
Concept explainers
(a)
Interpretation: To indicate whether hexokinase is associated with carbohydrate digestion or the glycolysis
Concept introduction: Carbohydrates are the
Carbohydrates are classified as monosaccharide, disaccharide, oligosaccharide, and polysaccharide. Monosaccharides are the simplest carbohydrate units that cannot be hydrolyzed further to give the smallest units. Disaccharides contain two monosaccharide units. Oligosaccharides contain 3 to 10 monosaccharide units. Polysaccharides contain many carbohydrate units that vary from 100 to 50,000 monosaccharide units.
In the glycolysis metabolic pathway, a glucose molecule breaks down and is converted into two pyruvate molecules along with the production of two ATP molecules and NADH coenzymes.
The block diagram to represent an overview of glycolysis is as follows:
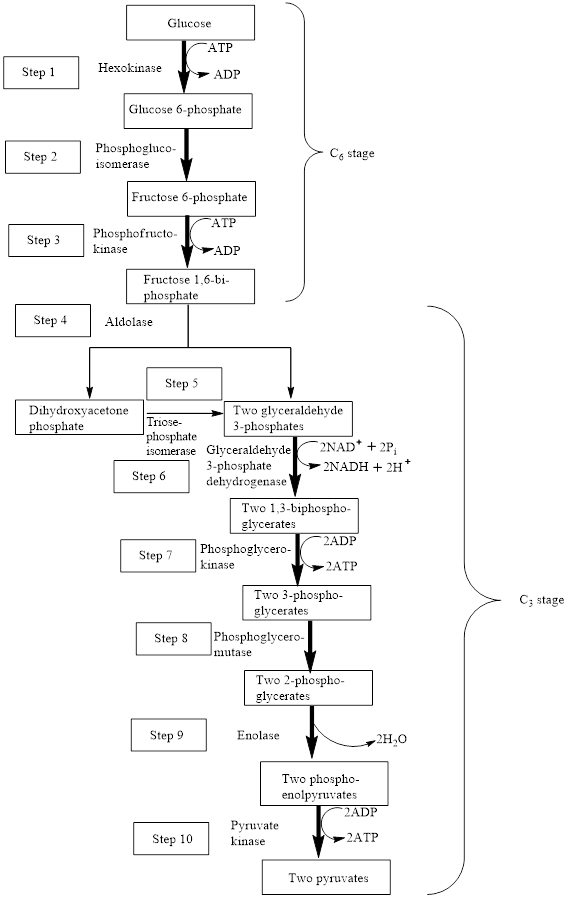
From the above diagram, it is concluded that in the overall process of glycolysis, two stages are present.
a) Steps 1 to 3 represents a six-carbon stage
b) Steps 4 to 10 represent a three-carbon stage
(b)
Interpretation: To indicate whether lactase is associated with carbohydrate digestion or the glycolysis metabolic pathway.
Concept introduction: Carbohydrates are the biomolecules composed of carbon, oxygen and hydrogen atoms. Carbohydrate molecules are joined together by glycosidic linkage.
Carbohydrates are classified as monosaccharide, disaccharide, oligosaccharide, and polysaccharide. Monosaccharides are the simplest carbohydrate units that cannot be hydrolyzed further to give the smallest units. Disaccharides contain two monosaccharide units. Oligosaccharides contain 3 to 10 monosaccharide units. Polysaccharides contain many carbohydrate units that vary from 100 to 50,000 monosaccharide units.
In the glycolysis metabolic pathway, a glucose molecule breaks down and is converted into two pyruvate molecules along with the production of two ATP molecules and NADH coenzymes.
The block diagram to represent an overview of glycolysis is as follows:

From the above diagram, it is concluded that in the overall process of glycolysis, two stages are present.
a) Steps 1 to 3 represents a six-carbon stage
b) Steps 4 to 10 represent a three-carbon stage
(c)
Interpretation: To indicate whether the hydrolysis reaction is associated with carbohydrate digestion or the glycolysis metabolic pathway.
Concept introduction: Carbohydrates are the biomolecules composed of carbon, oxygen and hydrogen atoms. Carbohydrate molecules are joined together by glycosidic linkage.
Carbohydrates are classified as monosaccharide, disaccharide, oligosaccharide, and polysaccharide. Monosaccharides are the simplest carbohydrate units that cannot be hydrolyzed further to give the smallest units. Disaccharides contain two monosaccharide units. Oligosaccharides contain 3 to 10 monosaccharide units. Polysaccharides contain many carbohydrate units that vary from 100 to 50,000 monosaccharide units.
In the glycolysis metabolic pathway, a glucose molecule breaks down and is converted into two pyruvate molecules along with the production of two ATP molecules and NADH coenzymes.
The block diagram to represent an overview of glycolysis is as follows:

From the above diagram, it is concluded that in the overall process of glycolysis, two stages are present.
a) Steps 1 to 3 represents a six-carbon stage
b) Steps 4 to 10 represent a three-carbon stage
(d)
Interpretation: To indicate whether the dehydration reaction is associated with carbohydrate digestion or the glycolysis metabolic pathway.
Concept introduction: Carbohydrates are the biomolecules composed of carbon, oxygen and hydrogen atoms. Carbohydrate molecules are joined together by glycosidic linkage.
Carbohydrates are classified as monosaccharide, disaccharide, oligosaccharide, and polysaccharide. Monosaccharides are the simplest carbohydrate units that cannot be hydrolyzed further to give the smallest units. Disaccharides contain two monosaccharide units. Oligosaccharides contain 3 to 10 monosaccharide units. Polysaccharides contain many carbohydrate units that vary from 100 to 50,000 monosaccharide units.
In the glycolysis metabolic pathway, a glucose molecule breaks down and is converted into two pyruvate molecules along with the production of two ATP molecules and NADH coenzymes.
The block diagram to represent an overview of glycolysis is as follows:

From the above diagram, it is concluded that in the overall process of glycolysis, two stages are present.
a) Steps 1 to 3 represents a six-carbon stage
b) Steps 4 to 10 represent a three-carbon stage
Want to see the full answer?
Check out a sample textbook solution
Chapter 13 Solutions
EBK ORGANIC AND BIOLOGICAL CHEMISTRY
- Synthesize 2-Ethyl-3-methyloxirane from dimethyl(propyl)sulfonium iodide using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Synthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIf possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forwardSynthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Indicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forwardWe mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forwardIndicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forward
- Indicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forwardIndicate the products obtained if (E)-2-butenal and 3-oxo-butanenitrile are mixed with sodium ethoxide in ethanol.arrow_forwardQuestion 3 (4 points), Draw a full arrow-pushing mechanism for the following reaction Please draw all structures clearly. Note that this intramolecular cyclization is analogous to the mechanism for halohydrin formation. COH Br + HBr Brarrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,



