
Concept explainers
(a)
Interpretation: To indicate whether B vitamin thiamin is involved in (1) glycolysis, (2) gluconeogenesis, (3) lactate fermentation, or (4) glycogenolysis as a cofactor.
Concept introduction: Vitamins are defined as the micronutrients that are needed in a small amount for the proper functioning of the metabolic activities in the organisms.
Cofactors are non-protein organic compounds that are used along with the enzymes and help to carry forward the reaction. Cofactors cannot perform on their own alone.
In the glycolysis metabolic pathway, a glucose molecule breaks down into two pyruvate molecules. In gluconeogenesis process, glucose is produced from non-carbohydrate substances. Glycogenolysis is the metabolic pathway that converts glycogen to
In the absence of oxygen, pyruvate is converted to lactate by lactate dehydrogenase enzymes in the human body. In this reaction, NADH is oxidized to
Niacin
(a)
Answer to Problem 13.117EP
None of the given processes includes B vitamin thiamin as a cofactor. B vitamin thiamin is needed as a cofactor in the conversion of pyruvate to
Explanation of Solution
B vitamin thiamin is encountered in the form of thiamin pyrophosphate (TPP) in the carbohydrate metabolism. TPP in not involved in glycolysis, gluconeogenesis, lactate fermentation, and glycogenolysis. Hence, none of the given processes includes vitamin thiamin as a cofactor.
Pyruvate is converted to
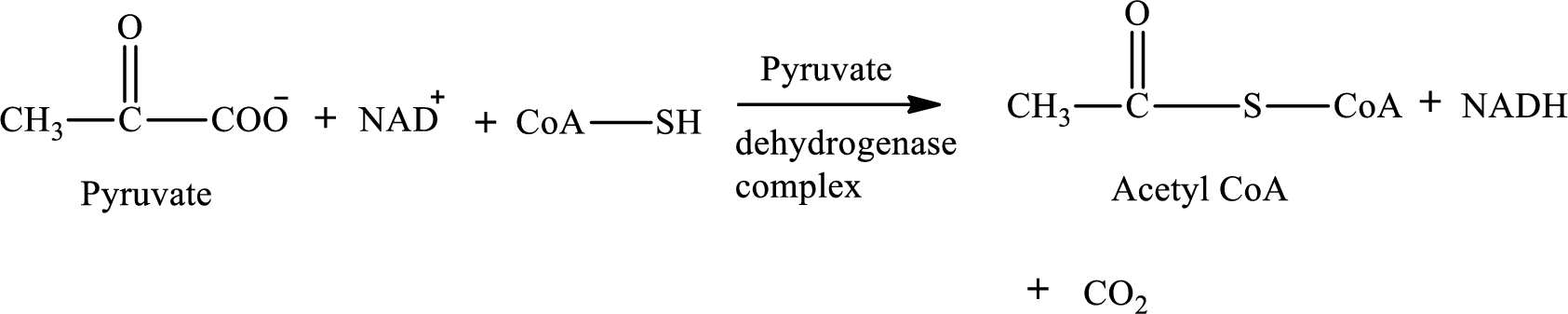
Pyruvate dehydrogenase complex contains three different enzymes. Each enzyme contains numerous subunits. The overall reaction requires FAD,
(b)
Interpretation: To indicate B vitamin riboflavin is involved in (1) glycolysis, (2) gluconeogenesis, (3) lactate fermentation, or (4) glycogenolysis as a cofactor.
Concept introduction: Vitamins are defined as the micronutrients that are needed in a small amount for the proper functioning of the metabolic activities in the organisms.
Cofactors are non-protein organic compounds that are used along with the enzymes and help to carry forward the reaction. Cofactors cannot perform on their own alone.
In the glycolysis metabolic pathway, a glucose molecule breaks down into two pyruvate molecules. In gluconeogenesis process, glucose is produced from non-carbohydrate substances. Glycogenolysis is the metabolic pathway that converts glycogen to
In the absence of oxygen, pyruvate is converted to lactate by lactate dehydrogenase enzymes in the human body. In this reaction, NADH is oxidized to
Niacin
(b)
Answer to Problem 13.117EP
None of the given processes includes vitamin riboflavin as a cofactor. B vitamin riboflavin is needed as a cofactor in the citric acid cycle.
Explanation of Solution
B vitamin riboflavin is encountered in the form of FAD(Flavin adenine dinucleotide) in the carbohydrate metabolism. FAD in not involved in glycolysis, gluconeogenesis, lactate fermentation, and glycogenolysis. Hence, none of the given processes includes B vitamin riboflavin as a cofactor.
The citric acid cycle is the third stage of the biochemical energy production process. The cycle includes the reactions in which the acetyl part of acetyl CoA is oxidized and leads to the formation of carbon dioxide and
(c)
Interpretation: To indicate whether B vitamin pantothenic acid is involved in (1) glycolysis, (2) gluconeogenesis, (3) lactate fermentation, or (4) glycogenolysis as a cofactor.
Concept introduction: Vitamins are defined as the micronutrients that are needed in a small amount for the proper functioning of the metabolic activities in the organisms.
Cofactors are non-protein organic compounds that are used along with the enzymes and help to carry forward the reaction. Cofactors cannot perform on their own alone.
In the glycolysis metabolic pathway, a glucose molecule breaks down into two pyruvate molecules. In gluconeogenesis process, glucose is produced from non-carbohydrate substances. Glycogenolysis is the metabolic pathway that converts glycogen to
In the absence of oxygen, pyruvate is converted to lactate by lactate dehydrogenase enzymes in the human body. In this reaction, NADH is oxidized to
Niacin
(c)
Answer to Problem 13.117EP
None of the given processes includes B vitamin pantothenic acid as a cofactor. B vitamin pantothenic acid is needed as a cofactor in the conversion of pyruvate to
Explanation of Solution
B vitamin pantothenic acid is encountered in the form of
Pyruvate is converted to
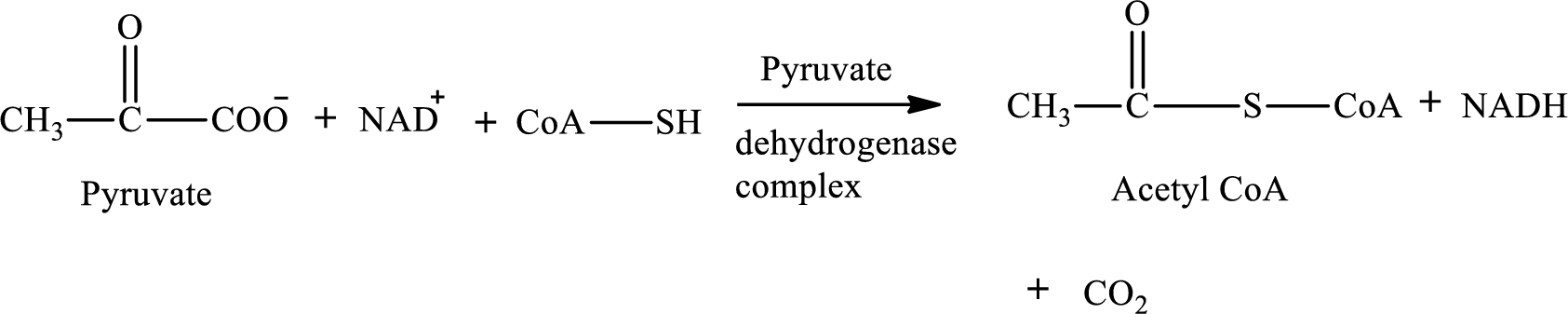
Pyruvate dehydrogenase complex contains three different enzymes. Each enzyme contains numerous subunits. The overall reaction requires FAD,
(d)
Interpretation: To indicate
Concept introduction: Vitamins are defined as the micronutrients that are needed in a small amount for the proper functioning of the metabolic activities in the organisms.
Cofactors are non-protein organic compounds that are used along with the enzymes and help to carry forward the reaction. Cofactors cannot perform on their own alone.
In the glycolysis metabolic pathway, a glucose molecule breaks down into two pyruvate molecules. In gluconeogenesis process, glucose is produced from non-carbohydrate substances. Glycogenolysis is the metabolic pathway that converts glycogen to
In the absence of oxygen, pyruvate is converted to lactate by lactate dehydrogenase enzymes in the human body. In this reaction, NADH is oxidized to
Niacin
Answer to Problem 13.117EP
Explanation of Solution
Want to see more full solutions like this?
Chapter 13 Solutions
EBK ORGANIC AND BIOLOGICAL CHEMISTRY
- I'm having trouble with converting lewis diagrams into VSEPR diagrams. I currently have this example of C2BrCl3 which I want to turn into a lewis structure, but I'm not sure what steps I need to do in order to do so. I have the table written down, however, there's two central atoms so what would I do? There seems to be 4 electron domains on the carbon atom and no lone pairs so it would seem like this shape would be tetrahedral. Here's what I have now. Thanks!arrow_forwardWe discussed the solid phase resin using in peptide synthesis. Provide a mechanism, for its formation. DRAW THE MECHANISM.arrow_forwardPlease help. Every time I've asked an expert in the past, it's been wrong :(arrow_forward
- Please help everysingle time ive asked in the past, the solution has been wrongarrow_forwardPlease helparrow_forward(a) 21.8 Name the following compounds. & (b) Br (e) O₂N. (h) H (c) Br (d) NH2 ☑N Br H ہیں Ph (g) OMe бл .0-0.e 21.9 Draw a structural formula for each compound. (a) 2,3-Dinitrotoluene (c) Diphenylmethanol (e) p-Nitroaniline (b) 3-Propylanisole (d) m-Propylphenol (f) Pentabromobenzenearrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





