
Concept explainers
(a)
Interpretation:
Glucose-6-phosphate is encountered in which of the four processes glycolysis, glycogenesis, glycogenolysis, and gluconeogenesis has to be indicated.
Concept introduction:
In the glycolysis
Gluconeogenesis is an eleven-step pathway in which glucose is produced from non-carbohydrate substances. Glycogenesis is the metabolic pathway that converts glucose-6-phosphate to glycogen. Glycogenolysis is the metabolic pathway that converts glycogen to glucose-6-phosphate.
Glucose-6-phosphate is an activated glucose molecule. The structure of glucose-6-phosphate is as follows:
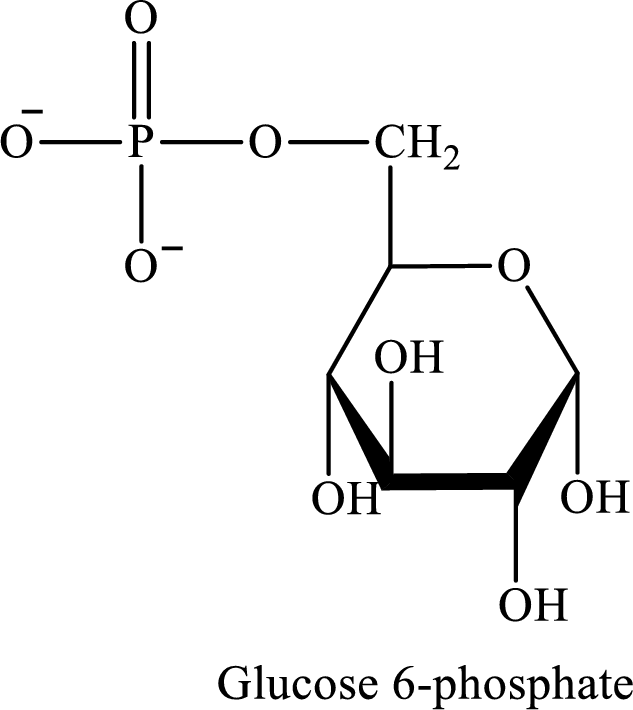
(b)
Interpretation:
Dihydroxyacetone phosphate is encountered in which of the four processes glycolysis, glycogenesis, glycogenolysis, and gluconeogenesis has to be indicated.
Concept introduction: In the glycolysis metabolic pathway, a glucose molecule breaks down into two pyruvate molecules. Two ATP molecules and NADH reduced coenzymes are produced in the glycolysis pathway.
Gluconeogenesis is an eleven-step pathway in which glucose is produced from non-carbohydrate substances. Glycogenesis is the metabolic pathway that converts glucose-6-phosphate to glycogen. Glycogenolysis is the metabolic pathway that converts glycogen to glucose-6-phosphate.
The structure of dihydroxyacetone phosphate is as follows:
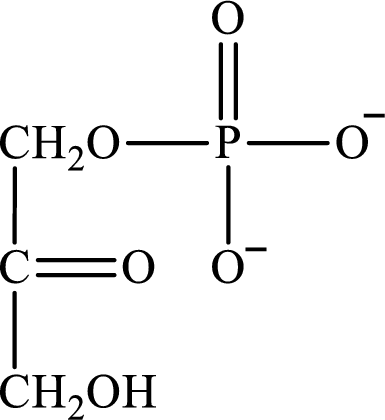
(c)
Interpretation:
Oxaloacetate is encountered in which of the four processes glycolysis, glycogenesis, glycogenolysis, and gluconeogenesis has to be indicated.
Concept introduction:
In the glycolysis metabolic pathway, a glucose molecule breaks down into two pyruvate molecules. Two ATP molecules and NADH reduced coenzymes are produced in the glycolysis pathway.
Gluconeogenesis is an eleven-step pathway in which glucose is produced from non-carbohydrate substances. Glycogenesis is the metabolic pathway that converts glucose-6-phosphate to glycogen. Glycogenolysis is the metabolic pathway that converts glycogen to glucose-6-phosphate.
The structure of oxaloacetate is as follows:
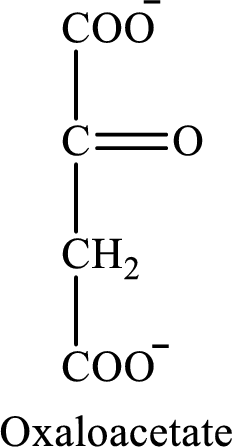
(d)
Interpretation:
UDP-glucose is encountered in which of the four processes glycolysis, glycogenesis, glycogenolysis, and gluconeogenesis has to be indicated.
Concept introduction:
In the glycolysis metabolic pathway, a glucose molecule breaks down into two pyruvate molecules. Two ATP molecules and NADH reduced coenzymes are produced in the glycolysis pathway.
Gluconeogenesis is an eleven-step pathway in which glucose is produced from non-carbohydrate substances. Glycogenesis is the metabolic pathway that converts glucose-6-phosphate to glycogen. Glycogenolysis is the metabolic pathway that converts glycogen to glucose-6-phosphate.
The structure of UDP-glucose is as follows:
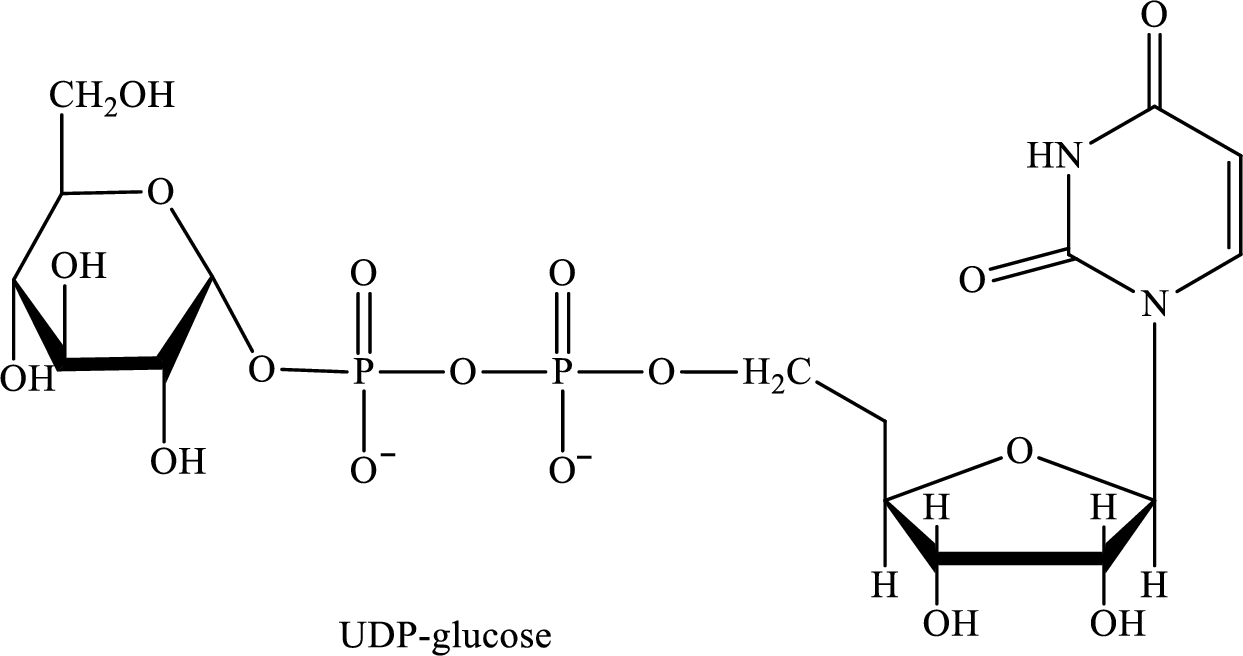
Want to see the full answer?
Check out a sample textbook solution
Chapter 13 Solutions
EBK ORGANIC AND BIOLOGICAL CHEMISTRY
- If possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forwardSynthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIndicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forward
- We mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forwardIndicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forwardIndicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forward
- Indicate the products obtained if (E)-2-butenal and 3-oxo-butanenitrile are mixed with sodium ethoxide in ethanol.arrow_forwardQuestion 3 (4 points), Draw a full arrow-pushing mechanism for the following reaction Please draw all structures clearly. Note that this intramolecular cyclization is analogous to the mechanism for halohydrin formation. COH Br + HBr Brarrow_forwardIndicate the products obtained if 2,2-dimethylpropanal and acetaldehyde are mixed with sodium ethoxide in ethanol.arrow_forward
- Indicate the products obtained if 2,2-dimethylpropanal and acetaldehyde are reacted with sodium ethoxide in ethanol.arrow_forward2,2-Dimethylpropanal and acetaldehyde are reacted with sodium ethoxide in ethanol. Indicate the products obtained.arrow_forwardAdd conditions above and below the arrow that turn the reactant below into the product below in a single transformationADS fint anditions 百 Abl res condinese NC ง Add on condtions 1.0 B H,N.arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,


