
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Chapter 1.3, Problem 1.12P
Interpretation Introduction
Interpretation:
The structural formulas for four esters with the molecular formula
Concept introduction:
Isomer: A molecule having same molecular formula with different chemical formula is called isomer.
Carboxylic ester:
A carbon atom is double-bonded to an oxygen atom
If the carbonyl carbon is attached with hydroxyl group is called as
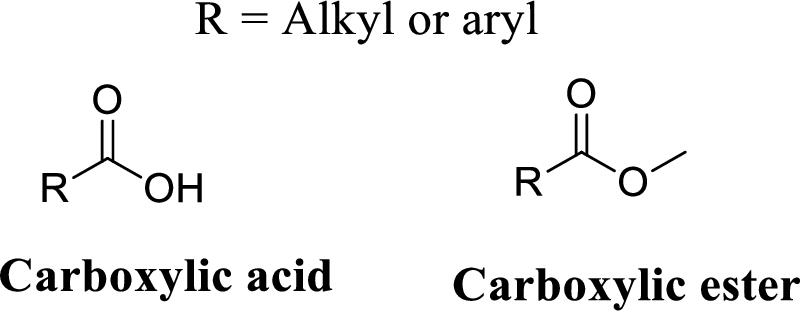
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Draw the Fischer projection of D-fructose.
Click and drag to start drawing a
structure.
Skip Part
Check
AP
14
tv
SC
F1
F2
80
F3
a
F4
!
2
#
3
CF
F5
75
Ax
MacBook Air
894
$
5olo
%
Λ
6 >
W
F6
K
F7
&
Consider this step in a radical reaction:
Y
What type of step is this? Check all that apply.
Draw the products of the step on the right-hand side of the drawing area
below. If more than one set of products is possible, draw any set.
Also, draw the mechanism arrows on the left-hand side of the drawing
area to show how this happens.
ionization
propagation
initialization
passivation
none of the above
22.16 The following groups are ortho-para directors.
(a)
-C=CH₂
H
(d)
-Br
(b)
-NH2
(c)
-OCHS
Draw a contributing structure for the resonance-stabilized cation formed during elec-
trophilic aromatic substitution that shows the role of each group in stabilizing the
intermediate by further delocalizing its positive charge.
22.17 Predict the major product or products from treatment of each compound with
Cl₁/FeCl₂-
OH
(b)
NO2
CHO
22.18 How do you account for the fact that phenyl acetate is less reactive toward electro-
philic aromatic substitution than anisole?
Phenyl acetate
Anisole
CH
(d)
Chapter 1 Solutions
Organic Chemistry
Ch. 1.1 - Prob. 1.1PCh. 1.2 - Prob. 1.2PCh. 1.2 - Judging from their relative positions in the...Ch. 1.2 - Classify each bond as nonpolar covalent or polar...Ch. 1.2 - Using the symbols and +, indicate the direction...Ch. 1.2 - Draw Lewis structures showing all valence...Ch. 1.2 - Draw Lewis structures for these ions and show...Ch. 1.3 - Draw Lewis structures and condensed structural...Ch. 1.3 - Prob. 1.9PCh. 1.3 - Prob. 1.10P
Ch. 1.3 - Prob. 1.11PCh. 1.3 - Prob. 1.12PCh. 1.4 - Predict all bond angles for these molecules. (a)...Ch. 1.5 - The geometry of carbon in diamond is tetrahedral,...Ch. 1.5 - Because of their spherical shape, C60 molecules...Ch. 1.5 - What best describes the CCC bond angles in C60? 1....Ch. 1.5 - Prob. 1.14PCh. 1.7 - Describe the bonding in these molecules in terms...Ch. 1.8 - Prob. 1.16PCh. 1.8 - Prob. 1.17PCh. 1.8 - Prob. 1.18PCh. 1.9 - Draw three contributing structures of the...Ch. 1.9 - What is the hybridization state of the circled...Ch. 1.9 - The molecule shown on the right in the example in...Ch. 1.9 - Prob. CQCh. 1.9 - The following structure is called imidazolium....Ch. 1 - Write the ground-state electron configuration for...Ch. 1 - Identify the atom that has each ground-state...Ch. 1 - Define valence shell and valence electron.Ch. 1 - How many electrons are in the valence shell of...Ch. 1 - Prob. 1.24PCh. 1 - Prob. 1.25PCh. 1 - Prob. 1.26PCh. 1 - Write Lewis structures for these compounds. Show...Ch. 1 - Write Lewis structures for these ions. Show all...Ch. 1 - Prob. 1.29PCh. 1 - Some of these structural formulas are incorrect...Ch. 1 - Following the rule that each atom of carbon,...Ch. 1 - Following are several Lewis structures showing all...Ch. 1 - Which statements are true about electronegativity?...Ch. 1 - Why does fluorine, the element in the upper right...Ch. 1 - Arrange the single covalent bonds within each set...Ch. 1 - Using the values of electronegativity given in...Ch. 1 - Prob. 1.37PCh. 1 - Use VSEPR to predict bond angles about each...Ch. 1 - Use VSEPR to predict bond angles about each atom...Ch. 1 - Use VSEPR to predict the geometry of these ions....Ch. 1 - Prob. 1.41PCh. 1 - Prob. 1.42PCh. 1 - What is the meaning of the term tertiary (3) when...Ch. 1 - What is the meaning of the term tertiary (3) when...Ch. 1 - Draw structural formulas for (a) The four primary...Ch. 1 - Draw structural formulas for the three tertiary...Ch. 1 - Prob. 1.47PCh. 1 - Identify the functional groups in each compound.Ch. 1 - Draw a three-dimensional representation for each...Ch. 1 - Tetrafluoroethylene, C2F4, is the starting...Ch. 1 - Which statements are true about resonance...Ch. 1 - Prob. 1.52PCh. 1 - Prob. 1.53PCh. 1 - Prob. 1.54PCh. 1 - Are the structures in each set valid contributing...Ch. 1 - State the orbital hybridization of each...Ch. 1 - Describe each highlighted bond in terms of the...Ch. 1 - Following is a structural formula of the...Ch. 1 - Draw a Lewis structure for methyl isocyanate,...Ch. 1 - What is the hybridization of the highlighted atoms...Ch. 1 - Using cartoon representations, draw a molecular...Ch. 1 - In what kind of orbitals do the lone-pair...Ch. 1 - Draw the delocalized molecular orbitals for the...Ch. 1 - Prob. 1.64APCh. 1 - Each compound contains both ions and covalent...Ch. 1 - Predict whether the carbon-metal bond in these...Ch. 1 - Prob. 1.67APCh. 1 - Phosphorus is immediately under nitrogen in the...Ch. 1 - Draw a Lewis structure for the azide ion, N3. (The...Ch. 1 - Cyanic acid, HOCN, and isocyanic acid, HNCO,...Ch. 1 - In Chapter 6, we study a group of organic cations...Ch. 1 - Many reactions involve a change in hybridization...Ch. 1 - Following is a structural formula of benzene,...Ch. 1 - Following are three contributing structures for...Ch. 1 - (a) Draw a Lewis structure for the ozone molecule,...Ch. 1 - The following two compounds are isomers; that is,...Ch. 1 - In future chapters, we will encounter...Ch. 1 - Prob. 1.78AP
Knowledge Booster
Similar questions
- Show how to convert ethyl benzene to (a) 2,5-dichlorobenzoic acid and (b) 2,4-dichlorobenzoic acid.arrow_forwardHelp me solve this problem. Thank you in advance.arrow_forward22.7 Predict the monoalkylated products of the following reactions with benzene. (a) AlCl3 Ya (b) AlCl3 (c) H3PO4 (d) 22.8 Think-Pair-Share AICI3 The reaction below is a common electrophilic aromatic substitution. SO3 H₂SO4 SO₂H (a) Draw the reaction mechanism for this reaction using HSO,+ as the electrophile. (b) Sketch the reaction coordinate diagram, where the product is lower in energy than the starting reactant. (c) Which step in the reaction mechanism is highest in energy? Explain. (d) Which of the following reaction conditions could be used in an electrophilic aro- matic substitution with benzene to provide substituted phenyl derivatives? (i) AICI3 HNO3 H₂SO4 K2Cr2O7 (iii) H₂SO4 (iv) H₂PO₁arrow_forward
- Is an acid-base reaction the only type of reaction that would cause leavening products to rise?arrow_forwardHelp me understand this! Thank you in advance.arrow_forward22.22 For each compound, indicate which group on the ring is more strongly activating and then draw a structural formula of the major product formed by nitration of the compound. Br CHO (a) CH3 (b) (c) CHO CH3 SO₂H (d) ☑ OCHS NO₂ (e) (f) CO₂H NHCOCH3 NHCOCH, (h) CHS 22.23 The following molecules each contain two aromatic rings. (b) 000-100- H3C (a) (c) Which ring in each undergoes electrophilic aromatic substitution more readily? Draw the major product formed on nitration.arrow_forward
- V Consider this step in a radical reaction: Br: ? What type of step is this? Check all that apply. Draw the products of the step on the right-hand side of the drawing area below. If more than one set of products is possible, draw any set. Also, draw the mechanism arrows on the left-hand side of the drawing area to show how this happens. ⚫ionization termination initialization neutralization none of the abc Explanation Check 80 Ο F3 F1 F2 2 F4 01 % do5 $ 94 #3 X 5 C MacBook Air 25 F5 F6 66 ©2025 ˇ F7 29 & 7 8arrow_forwardShow how to convert ethyl benzene to (a) 2,5-dichlorobenzoic acid and (b) 2,4-dichlorobenzoic acid.arrow_forwardno aiarrow_forward
- Polymers may be composed of thousands of monomers. Draw three repeat units (trimer) of the polymer formed in this reaction. Assume there are hydrogen atoms there are hydrogen atoms on the two ends of the trimer. Ignore inorganic byproducts.arrow_forwardDraw a tetramer if this alternating copolymer pleasearrow_forwardDraw the monomers required to synthesize this condensation polymer.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co