
Concept explainers
(a)
Interpretation:
Lewis structures have to be drawn for the
Concept introduction:
Lewis structure: The Lewis structure is based on the concept of the octet rule so that the electrons shared in each atom should have 8 electrons in its outer shell. Sometimes the
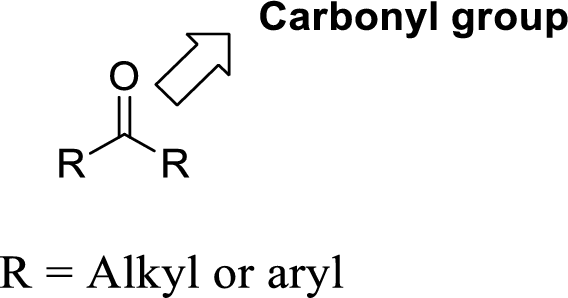
Carbonyl group:
A carbon atom is double-bonded to an oxygen atom
(b)
Interpretation:
Lewis structures have to be drawn for the functional group and valence electrons have to be shown.
Concept introduction:
Lewis structure: The Lewis structure is based on the concept of the octet rule so that the electrons shared in each atom should have 8 electrons in its outer shell. Sometimes the chemical bonding of a molecule cannot be represented using a single Lewis structure. In these cases, the chemical bonding are described by delocalization of electrons and is known as resonance. All the possible resonance structures are imaginary whereas the resonance hybrid is real. These structures will differ only in the arrangement of the electrons not in the relative position of the atomic nuclei.
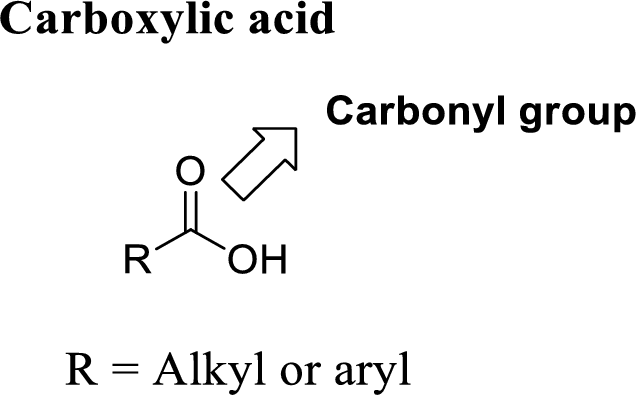
A carbon atom is double-bonded to an oxygen atom
If the carbonyl carbon is attached with hydroxyl group is called as carboxylic acid.
(c)
Interpretation:
Lewis structures have to be drawn for the functional group and valence electrons have to be shown.
Concept introduction:
Lewis structure: The Lewis structure is based on the concept of the octet rule so that the electrons shared in each atom should have 8 electrons in its outer shell. Sometimes the chemical bonding of a molecule cannot be represented using a single Lewis structure. In these cases, the chemical bonding are described by delocalization of electrons and is known as resonance. All the possible resonance structures are imaginary whereas the resonance hybrid is real. These structures will differ only in the arrangement of the electrons not in the relative position of the atomic nuclei.
Hydroxyl group:
Alcohol:
The compound contains hydroxyl
Example is given below
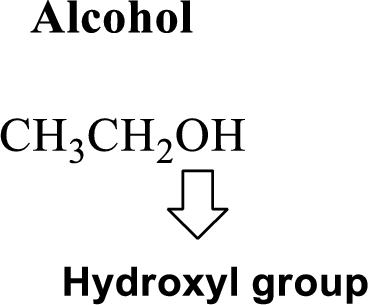
(d)
Interpretation:
Lewis structures have to be drawn for the functional group and valence electrons have to be shown.
Concept introduction:
Lewis structure: The Lewis structure is based on the concept of the octet rule so that the electrons shared in each atom should have 8 electrons in its outer shell. Sometimes the chemical bonding of a molecule cannot be represented using a single Lewis structure. In these cases, the chemical bonding are described by delocalization of electrons and is known as resonance. All the possible resonance structures are imaginary whereas the resonance hybrid is real. These structures will differ only in the arrangement of the electrons not in the relative position of the atomic nuclei.
Ester group:
A carbon atom is double-bonded to an oxygen atom
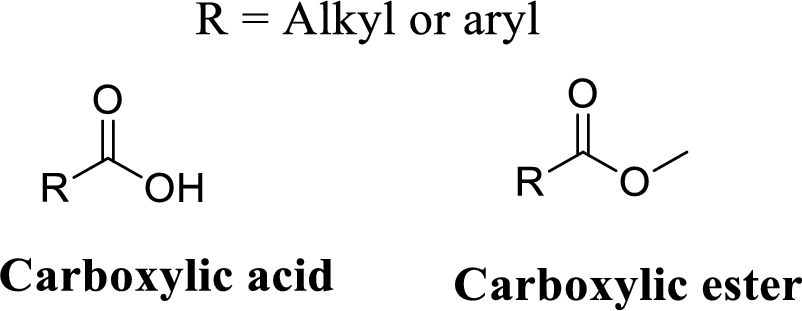
(e)
Interpretation:
Lewis structures have to be drawn for the functional group and valence electrons have to be shown.
Concept introduction:
Lewis structure: The Lewis structure is based on the concept of the octet rule so that the electrons shared in each atom should have 8 electrons in its outer shell. Sometimes the chemical bonding of a molecule cannot be represented using a single Lewis structure. In these cases, the chemical bonding are described by delocalization of electrons and is known as resonance. All the possible resonance structures are imaginary whereas the resonance hybrid is real. These structures will differ only in the arrangement of the electrons not in the relative position of the atomic nuclei.
Amide:
A carbon atom is double-bonded to an oxygen atom
If the carbonyl carbon is attached with nitrogen is called as amide.

Trending nowThis is a popular solution!

Chapter 1 Solutions
Organic Chemistry
- Consider the reaction below to answer the following questions. 0 0 25 PS ES 1919sds-III msx H H + 5% NaOCH 3, CH3OHA O CH₂OH Jeiniog 2E1 gniwool of mor]. Ignibuloni 9vil 19 A B 11 >buoqm gniwollol so dass 101 tomboy boo-11Coble or to r ton auch i viw ninlaxs, noitsausbroo 152 lobla ogsbau ton 250b br A. Which carbonyl compound functions as the electrophile in this reaction? B. Draw the structure of the enolate ion that is generated during the course of this reaction. C. This reaction is an example of: a. a mixed Claisen condensation. b. C. d. a Dieckman condensation. a Michael reaction. a mixed aldol reaction. HD HDarrow_forwardConsider the reaction below to answer the following questions: 847 Acetoacetic ester can be prepared by the Claisen self-condensation reaction of ethyl acetate. H₁C 0 H 0 IL 유 || OCH2CH3 1. NaOEt, EtOH C 2. H₂O H3C CH₂ Cold not tobizmo. S OCH2CH3 A. Write the complete stepwise mechanism for this reaction. Show all electron flow with arrows and draw all intermediate structures. B. Ethyl acetate can be prepared from ethanol as the only organic starting material. Show all reagents and structures for all intermediates in this preparation. C. Give the structures of the ester precursors for the following Claisen condensation product and formulate the reaction. ou OELarrow_forwardA. What is the correct structure for a-D-glucopyranose? CH₂OH a HO HO- OH b HO HO- OH HOH₂C OH OH OH CH₂OH HO C. HO HO- OH OH CH₂OH OH OH B. Draw structures for the products you would expect to obtain from reaction of B-D-galactopyranose with each of the following reagents. Be sure to include all relevant stereochemistry. [FOUR only] A. CH, Ag₂O B. warm dilute HNO3 C. (CH3CO)20, pryridine D. NaBH in H₂O E. CH₂OH, HCI F. Br₂, H₂O HO CH₂OH HO- OH OH B-D-galactopyranosearrow_forward
- Give the major organic product(s) for each of the following reactions or reaction sequences. CH₂CN 5% NaOEt, EIOH سجد سی . بلی H 1. NaOCH, CH,OH CH3 OCH3 2 H₂O*arrow_forwardDraw the structures for each of the intermediates in the boxes provided for the synthesis below. 004 HNO F HO CHCO) D Dydre R.SO. 1.1 NO fe HO H.SO. 2. CC1 NOH HO MCL HNO, H.50.arrow_forward. Each of the following compounds can be prepared by a mixed aldol condensation reaction. Give the Cructures of the aldehyde and/or ketone precursors for each aldol product and formulate the reaction. 0 CH=CHCCH 3. Ph 1arrow_forward
- . Consider the reaction below to answer the following question: H NaOEt H BOH بلی H + H₂O A. Write the complete stepwise mechanism for the reaction above. Show all intermediate structures and all electron flow with arrows. B. This reaction is an example of: an intramolecular aldol condensation a. an intramolecular Claisen condensation b. C. d. a Robinson annulation a Michael reaction C. The product of this reaction is: a. b. C. d. a ẞ. y-unsaturated aldehyde an a, B-unsaturated ketone an a, B-unsaturated aldehyde an enolarrow_forwardClassify each of the following nitrogen atoms in the following compounds as primary, secondary, tiary, or quaternary. A. B. C. CH3 HO-CHCHNHCH3 ephedrine CH CHCH3 amphetamine NH₂ D. CF H3C CH3 mapiquat chloride HO fexofenadine OH H3C CH3 CO₂Harrow_forward.. Name each of the following compounds by IUPAC rules. [three Only] A. 0 B. C. Cl NH₂ OCH N CH3 NHCH2CH3 D. CH O₁₂N NH₂arrow_forward
- Draw the structure of the aldol self condensation product for each of the following compounds if a compound does not under go aldol self condensation explain whyarrow_forwardIndicate how the addition of a nucleophile :NuH2 to a ketone R-CO-R occurs.arrow_forwardIndicate the compound that gives us a primary carbocation and that gives us a secondary carbocation: R-CHO and R-CO-Rarrow_forward
 Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
