
Concept explainers
Draw a three-dimensional representation for each molecule. Indicate which ones have a dipole moment and in what direction it is pointing.
- (a) CH3F
- (b) CH2Cl2
- (c) CH2ClBr
- (d) CFCl3
- (e) CCl4
- (f) CH2=CCl2
- (g) CH2=CHCl
- (h) HC≡C—C≡CH
- (i) CH3C≡N
- (j) (CH3)2C=O
- (k) BrCH=CHBr (two answers)
(a)
Interpretation:
The Three-dimensional representation for the molecule has to be drawn and dipole movement, the direction of its dipole moment has to be identified.
Concept introduction:
Polar Molecules:
Polar Molecules contains partial positive and partial negative charge in the molecule due its electronegativity difference between the molecules.
Dipole moment:
The charge separation of the molecule produces dipole moment. Dipole moment arises between two ions in an ionic bond or covalent bond.
Dipole moment depends on the differences in electronegativity of the atom in the molecule. The electronegativity is more, the larger the dipole moment. The dipole moment is calculated from the polarity of the molecule.
Explanation of Solution
The given compound is shown below,
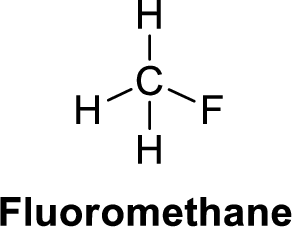
Fluoromethane is polar molecule because it has more electronegativity fluorine atom. The structure of fluoromethane is given below,

Fluorine is more electronegative than carbon and hydrogen. Carbon is more electronegative than hydrogen.
The direction of dipole moment in dichloromethane is given below,

The Three-dimensional representation for the molecule is shown below,
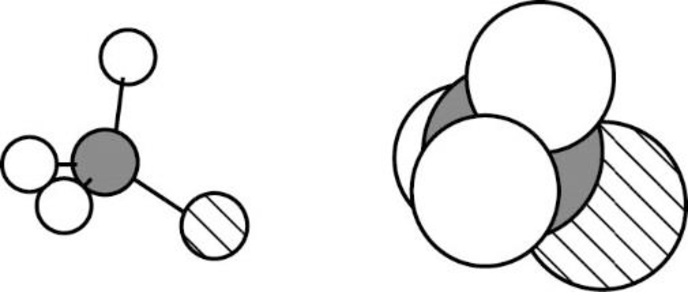
Figure 1
(b)
Interpretation:
Three-dimensional representation for the molecule has to be drawn and dipole movement, the direction of its dipole moment has to be identified.
Concept introduction:
Polar Molecules:
Polar Molecules contains partial positive and partial negative charge in the molecule due its electronegativity difference between the molecules.
Dipole moment:
The charge separation of the molecule produces dipole moment. Dipole moment arises between two ions in an ionic bond or covalent bond.
Dipole moment depends on the differences in electronegativity of the atom in the molecule. The electronegativity is more, the larger the dipole moment. The dipole moment is calculated from the polarity of the molecule.
Explanation of Solution
The given compound is shown below,
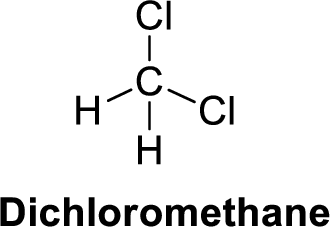
Dichloromethane is polar molecule because it has more electronegativity chlorine atom. The structure of dichloromethane is given below,

Chlorine is more electronegative than carbon and hydrogen. Carbon is more electronegative than hydrogen.
The direction of dipole moment in dichloromethane is given below,
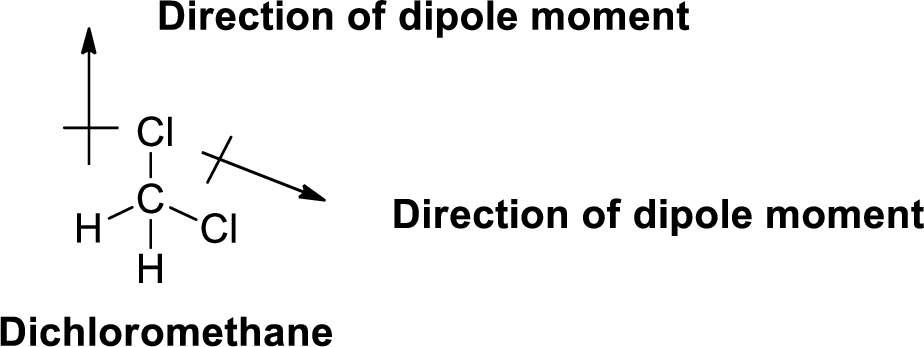
The Three-dimensional representation for the molecule is shown below,
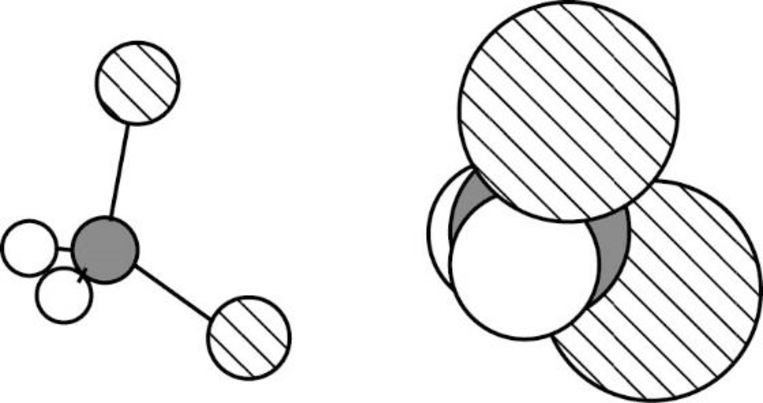
Figure 2
(c)
Interpretation:
Three-dimensional representation for the molecule has to be drawn and dipole movement, the direction of its dipole moment has to be identified.
Concept introduction:
Polar Molecules:
Polar Molecules contains partial positive and partial negative charge in the molecule due its electronegativity difference between the molecules.
Dipole moment:
The charge separation of the molecule produces dipole moment. Dipole moment arises between two ions in an ionic bond or covalent bond.
Dipole moment depends on the differences in electronegativity of the atom in the molecule. The electronegativity is more, the larger the dipole moment. The dipole moment is calculated from the polarity of the molecule.
Explanation of Solution
The given compound is shown below,
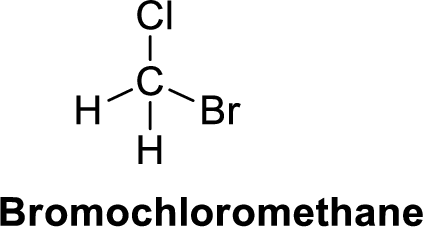
Bromochloromethane is polar molecule because it has more electronegativity chlorine and bromine atom. The structure of Bromochloromethane is given below,

Chlorine is more electronegative than carbon and hydrogen, similarly bromine is more electronegative than carbon and hydrogen. Carbon is more electronegative than hydrogen.
The direction of dipole moment in Bromochloromethane is given below,
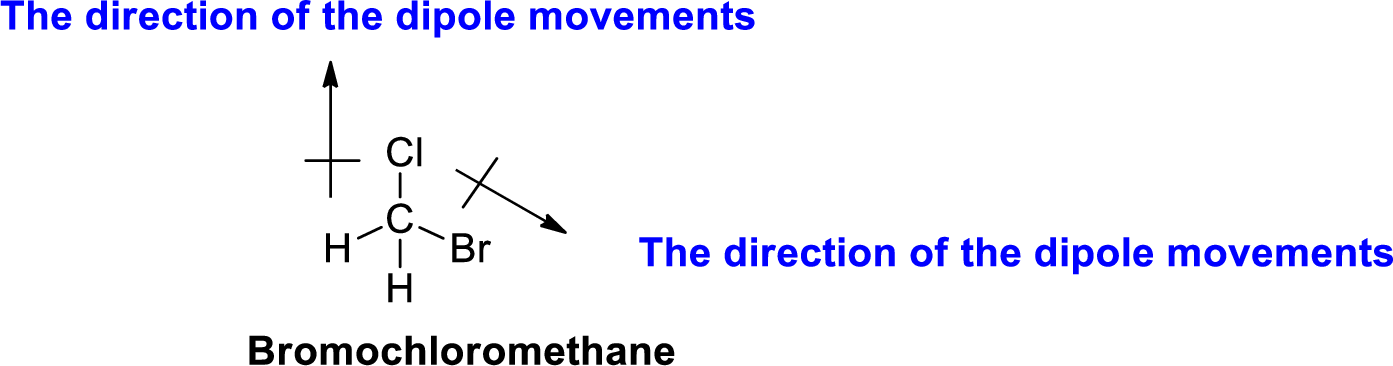
The Three-dimensional representation for the molecule is shown below,
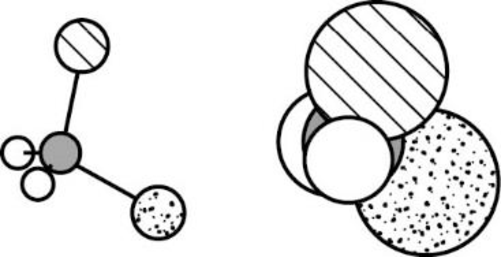
Figure 3
(d)
Interpretation:
Three-dimensional representation for the molecule has to be drawn and dipole movement, the direction of its dipole moment has to be identified.
Concept introduction:
Polar Molecules:
Polar Molecules contains partial positive and partial negative charge in the molecule due its electronegativity difference between the molecules.
Dipole moment:
The charge separation of the molecule produces dipole moment. Dipole moment arises between two ions in an ionic bond or covalent bond.
Dipole moment depends on the differences in electronegativity of the atom in the molecule. The electronegativity is more, the larger the dipole moment. The dipole moment is calculated from the polarity of the molecule.
Explanation of Solution
The given compound is shown below,
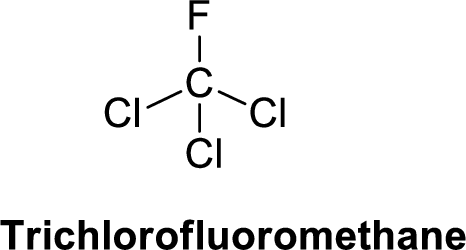
Trichlorofluoromethane is polar molecule because it has more electronegativity fluorine atom and chlorine atom. The structure of Trichlorofluoromethane is given below,
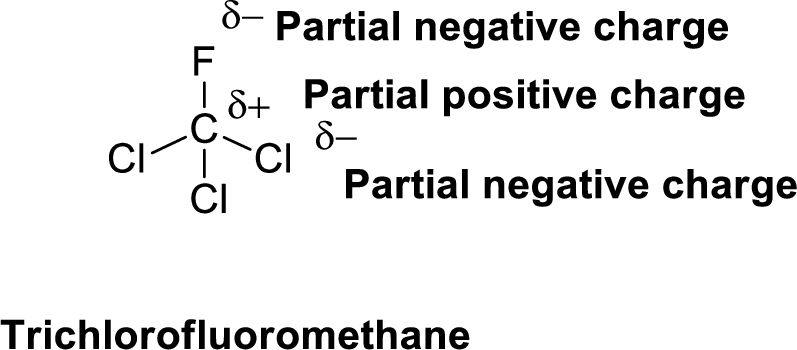
Fluorine is more electronegative than chlorine and carbon, similarly chlorine is more electronegative than carbon.
The direction of dipole moment in Trichlorofluoromethane is given below,
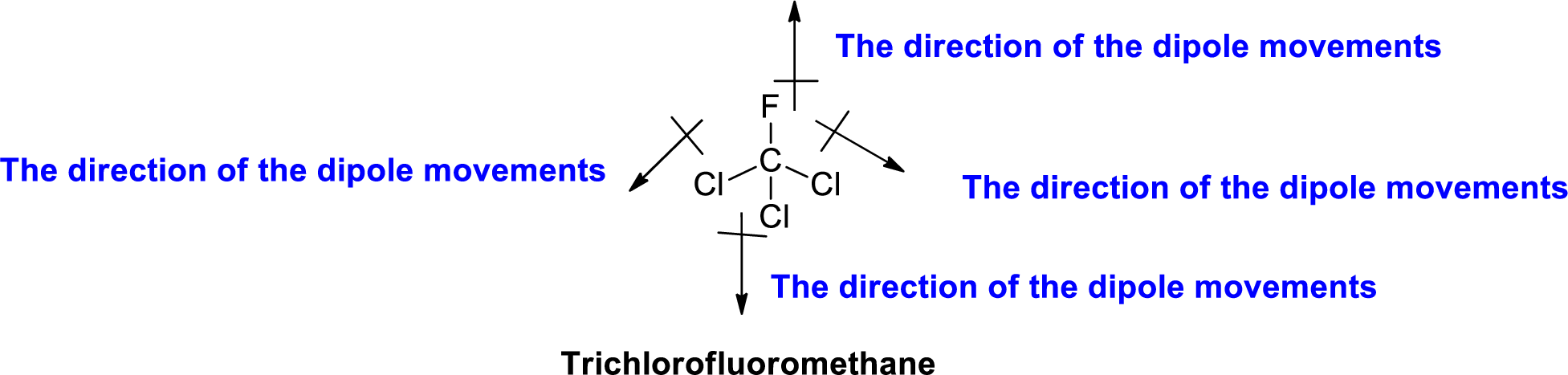
The Three-dimensional representation for the molecule is shown below,
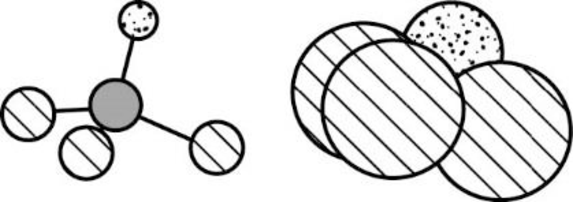
Figure 4
(e)
Interpretation:
Three-dimensional representation for the molecule has to be drawn and dipole movement, the direction of its dipole moment has to be identified.
Concept introduction:
Polar Molecules:
Polar Molecules contains partial positive and partial negative charge in the molecule due its electronegativity difference between the molecules.
Dipole moment:
The charge separation of the molecule produces dipole moment. Dipole moment arises between two ions in an ionic bond or covalent bond.
Dipole moment depends on the differences in electronegativity of the atom in the molecule. The electronegativity is more, the larger the dipole moment. The dipole moment is calculated from the polarity of the molecule.
Explanation of Solution
The given compound is shown below,
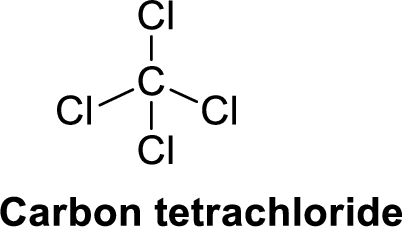
Carbon tetrachloride is polar molecule because it has more electronegativity chlorine. The structure of Carbon tetrachloride is given below,
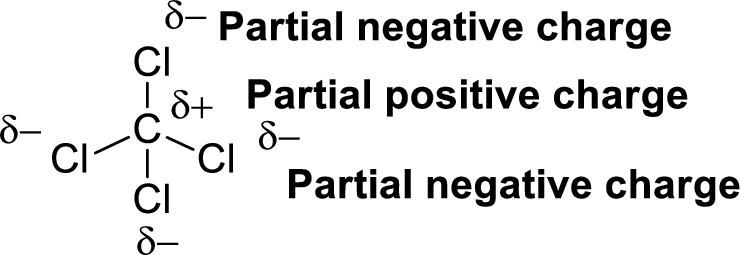
Chlorine is more electronegative than carbon. The direction of dipole moment in Carbon tetrachloride is given below,
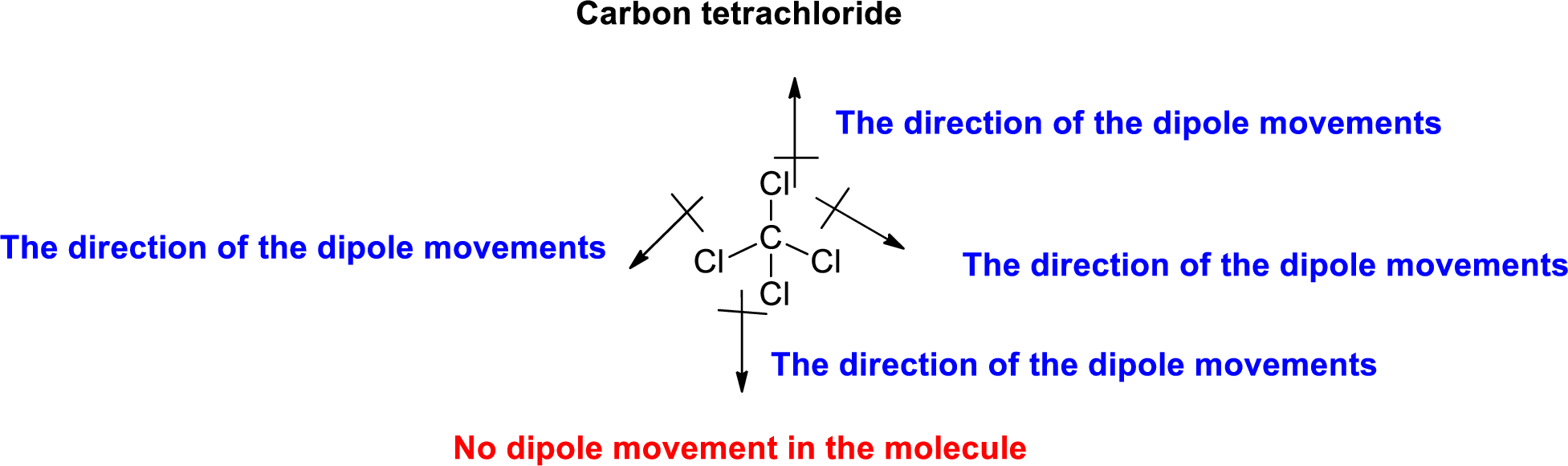
The Three-dimensional representation for the molecule is shown below,
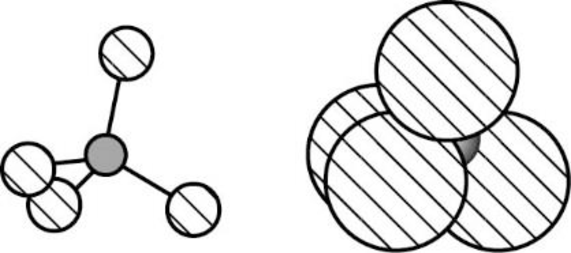
Figure 5
(f)
Interpretation:
Three-dimensional representation for the molecule has to be drawn and dipole movement, the direction of its dipole moment has to be identified.
Concept introduction:
Polar Molecules:
Polar Molecules contains partial positive and partial negative charge in the molecule due its electronegativity difference between the molecules.
Dipole moment:
The charge separation of the molecule produces dipole moment. Dipole moment arises between two ions in an ionic bond or covalent bond.
Dipole moment depends on the differences in electronegativity of the atom in the molecule. The electronegativity is more, the larger the dipole moment. The dipole moment is calculated from the polarity of the molecule.
Explanation of Solution
The given compound is shown below,
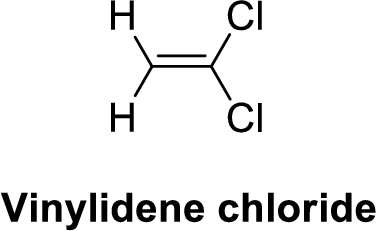
Vinylidene chloride is polar molecule because it has more electronegativity chlorine. The structure of Vinylidene chloride is given below,
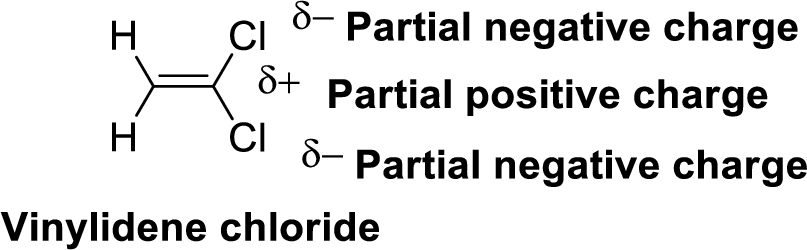
Chlorine is more electronegative than carbon and hydrogen, similarly Carbon is more electronegative than hydrogen.
The direction of dipole moment in Vinylidene chloride is given below,

The Three-dimensional representation for the molecule is shown below,
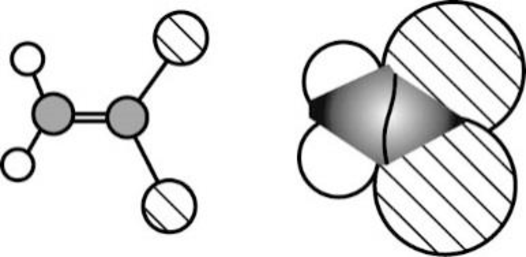
Figure 6
(g)
Interpretation:
Three-dimensional representation for the molecule has to be drawn and dipole movement, the direction of its dipole moment has to be identified.
Concept introduction:
Polar Molecules:
Polar Molecules contains partial positive and partial negative charge in the molecule due its electronegativity difference between the molecules.
Dipole moment:
The charge separation of the molecule produces dipole moment. Dipole moment arises between two ions in an ionic bond or covalent bond.
Dipole moment depends on the differences in electronegativity of the atom in the molecule. The electronegativity is more, the larger the dipole moment. The dipole moment is calculated from the polarity of the molecule.
Explanation of Solution
The given compound is shown below,
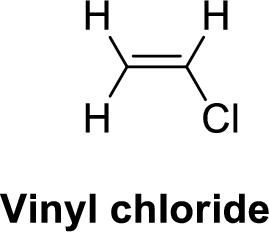
Vinyl chloride is polar molecule because it has more electronegativity chlorine. The structure of Vinyl chloride is given below,

Chlorine is more electronegative than carbon and hydrogen, similarly Carbon is more electronegative than hydrogen.
The direction of dipole moment in Vinyl chloride is given below,

The Three-dimensional representation for the molecule is shown below,
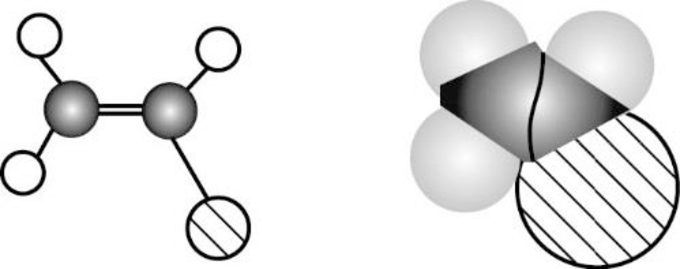
Figure 7
(h)
Interpretation:
Three-dimensional representation for the molecule has to be drawn and dipole movement, the direction of its dipole moment has to be identified.
Concept introduction:
Polar Molecules:
Polar Molecules contains partial positive and partial negative charge in the molecule due its electronegativity difference between the molecules.
Dipole moment:
The charge separation of the molecule produces dipole moment. Dipole moment arises between two ions in an ionic bond or covalent bond.
Dipole moment depends on the differences in electronegativity of the atom in the molecule. The electronegativity is more, the larger the dipole moment. The dipole moment is calculated from the polarity of the molecule.
Explanation of Solution
The given compound is non-polar molecule. The structure of compound is given below,
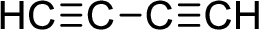
Carbon is more electronegative than hydrogen.
The direction of dipole moment in of the given compound is given below,
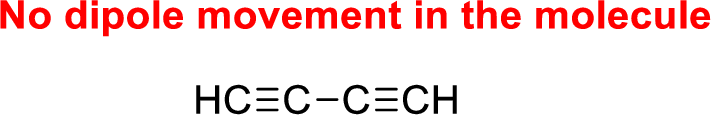
The Three-dimensional representation for the molecule is shown below,
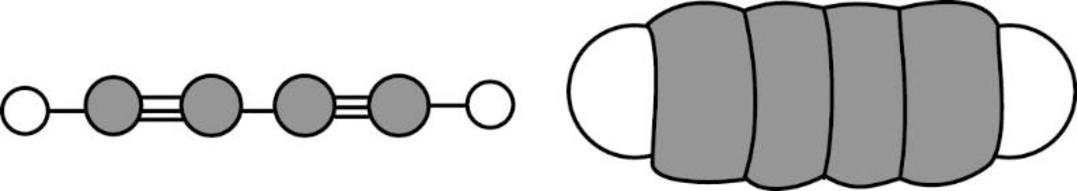
Figure 8
(i)
Interpretation:
Three-dimensional representation for the molecule has to be drawn and dipole movement, the direction of its dipole moment has to be identified.
Concept introduction:
Polar Molecules:
Polar Molecules contains partial positive and partial negative charge in the molecule due its electronegativity difference between the molecules.
Dipole moment:
The charge separation of the molecule produces dipole moment. Dipole moment arises between two ions in an ionic bond or covalent bond.
Dipole moment depends on the differences in electronegativity of the atom in the molecule. The electronegativity is more, the larger the dipole moment. The dipole moment is calculated from the polarity of the molecule.
Explanation of Solution
The given compound is shown below,
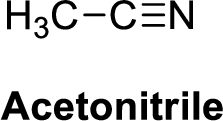
Acetonitrile is polar molecule because it has more electronegativity nitrogen atom. The structure of Acetonitrile is given below,
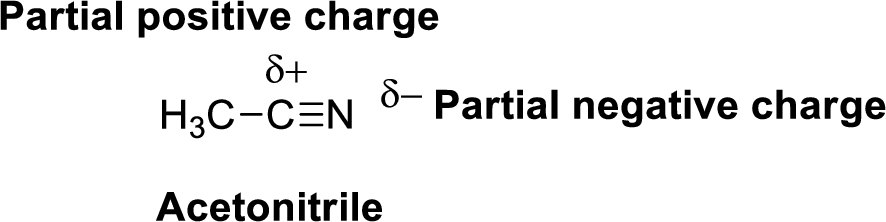
Nitrogen is more electronegative than carbon and hydrogen, similarly Carbon is more electronegative than hydrogen.
The direction of dipole moment in acetonitrile is given below,

The Three-dimensional representation for the molecule is shown below,
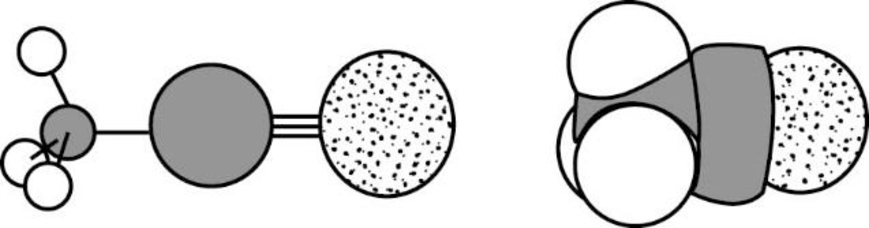
Figure 9
(j)
Interpretation:
Three-dimensional representation for the molecule has to be drawn and dipole movement, the direction of its dipole moment has to be identified.
Concept introduction:
Polar Molecules:
Polar Molecules contains partial positive and partial negative charge in the molecule due its electronegativity difference between the molecules.
Dipole moment:
The charge separation of the molecule produces dipole moment. Dipole moment arises between two ions in an ionic bond or covalent bond.
Dipole moment depends on the differences in electronegativity of the atom in the molecule. The electronegativity is more, the larger the dipole moment. The dipole moment is calculated from the polarity of the molecule.
Explanation of Solution
The given compound is shown below,
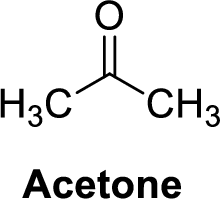
Acetone is polar molecule because it has more electronegativity oxygen atom. The structure of acetone is given below,
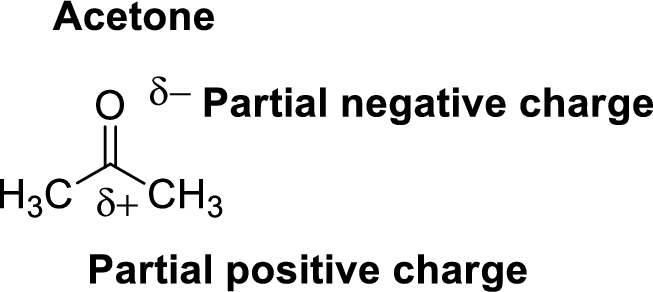
Oxygen is more electronegative than carbon and hydrogen, similarly Carbon is more electronegative than hydrogen.
The direction of dipole moment in acetone is given below,
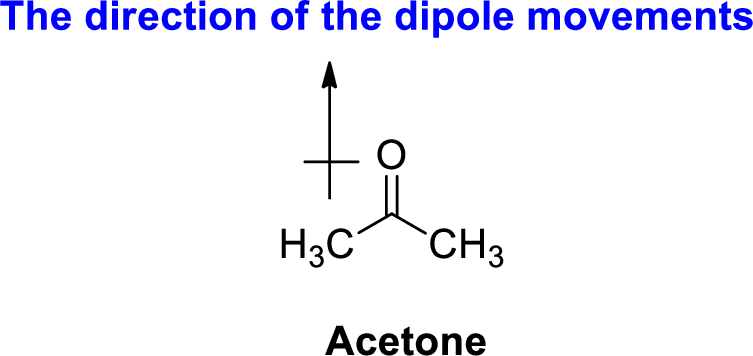
The Three-dimensional representation for the molecule is shown below,
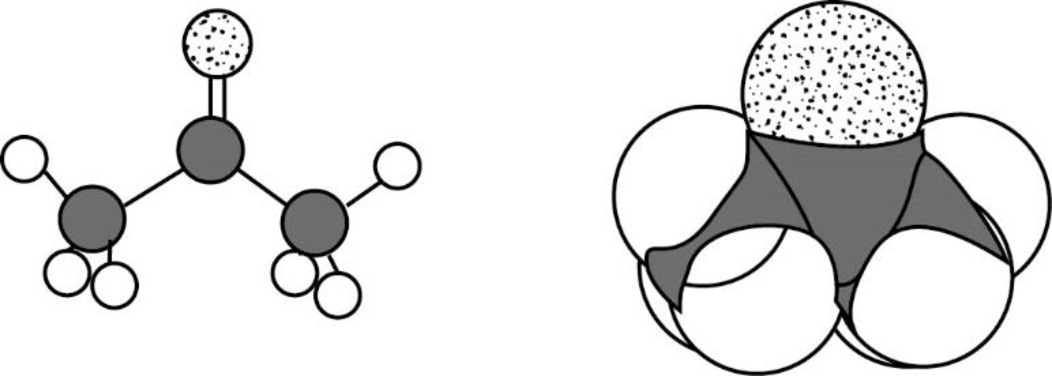
Figure 10
(k)
Interpretation:
Three-dimensional representation for the molecule has to be drawn and dipole movement, the direction of its dipole moment has to be identified.
Concept introduction:
Polar Molecules:
Polar Molecules contains partial positive and partial negative charge in the molecule due its electronegativity difference between the molecules.
Dipole moment:
The charge separation of the molecule produces dipole moment. Dipole moment arises between two ions in an ionic bond or covalent bond.
Dipole moment depends on the differences in electronegativity of the atom in the molecule. The electronegativity is more, the larger the dipole moment. The dipole moment is calculated from the polarity of the molecule.
Explanation of Solution
The given compound 1,2-Dibromoethylene, therefore it has two form
- (i) Cis 1,2-Dibromoethylene
- (ii) Trans- 1,2-Dibromoethylene
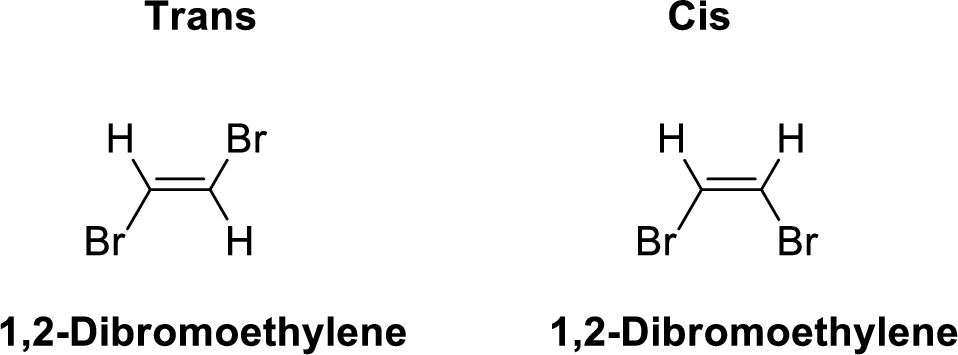
1,2-Dibromoethylene is polar molecule because it has more electronegativity bromine atom. The structure of 1,2-Dibromoethylene is given below,
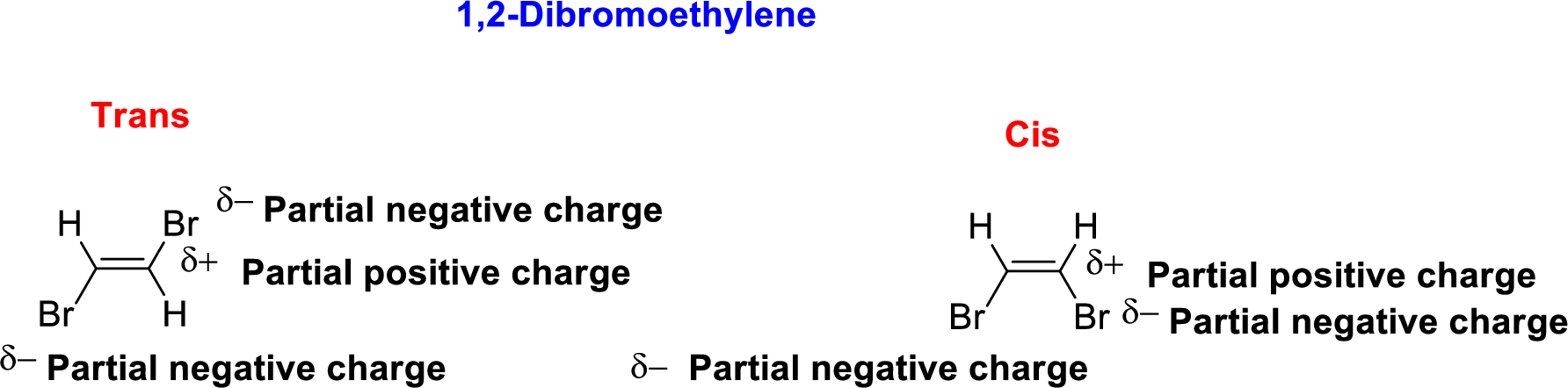
Bromine is more electronegative than carbon and hydrogen, similarly Carbon is more electronegative than hydrogen.
The direction of dipole moment in 1,2-Dibromoethylene is given below,
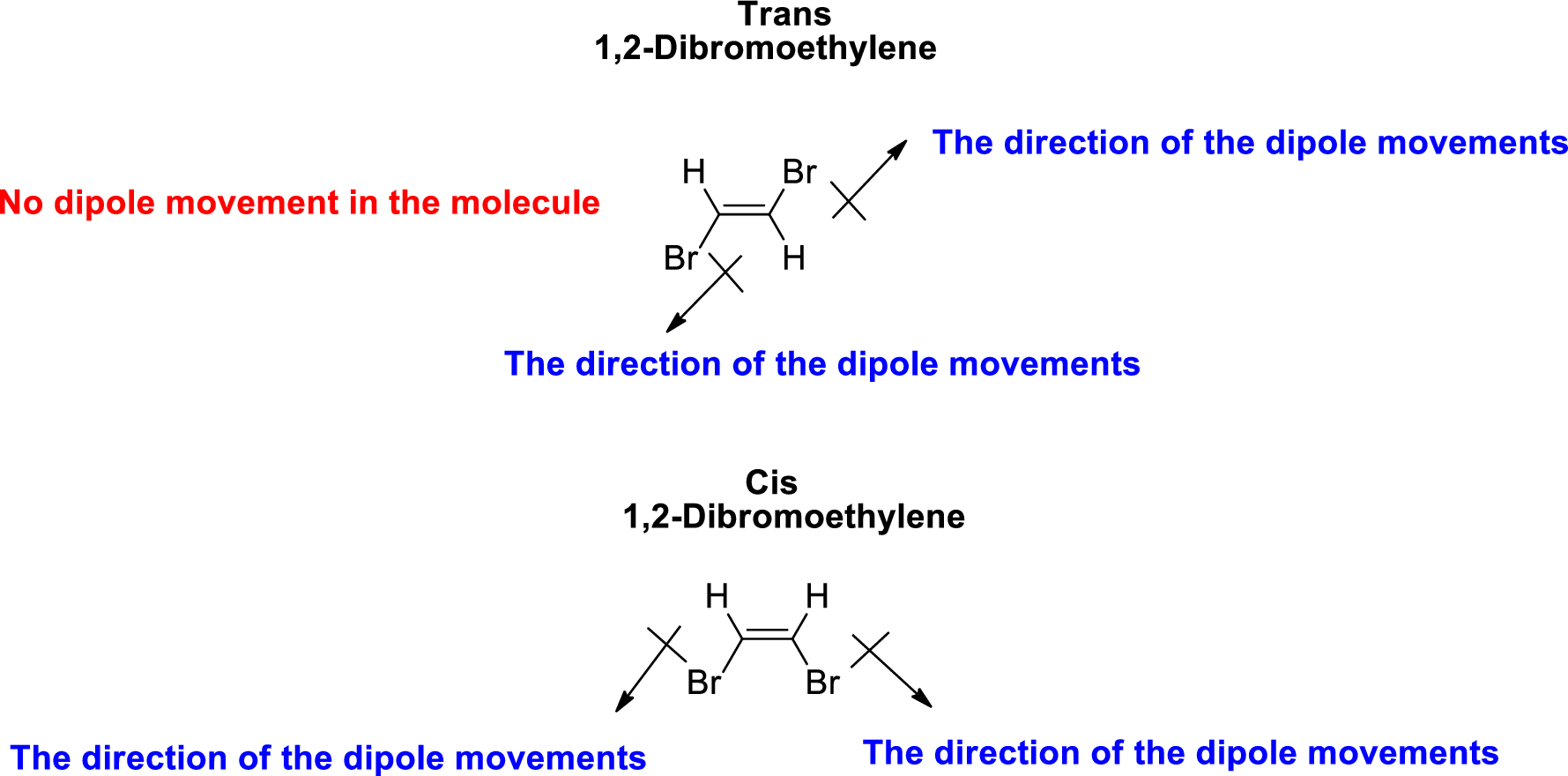
The Three-dimensional representation for the molecule is shown below,
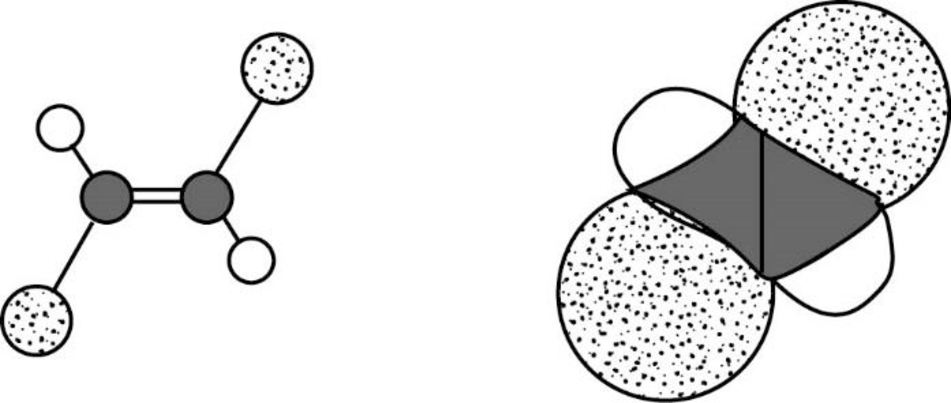
Figure 11
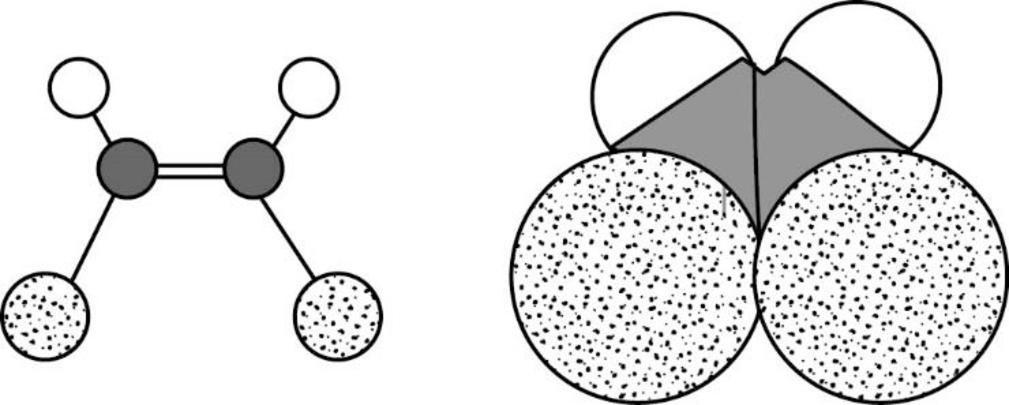
Figure 12
Want to see more full solutions like this?
Chapter 1 Solutions
Organic Chemistry
- 6.arrow_forward0/5 alekscgi/x/sl.exe/1o_u-IgNglkr7j8P3jH-IQs_pBaHhvlTCeeBZbufuBYTi0Hz7m7D3ZcSLEFovsXaorzoFtUs | AbtAURtkqzol 1HRAS286, O States of Matter Sketching a described thermodynamic change on a phase diagram The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 3 pressure (atm) + 0- 0 5+ 200 temperature (K) 400 Explanation Check X 0+ F3 F4 F5 F6 F7 S 2025 McGraw Hill LLC All Rights Reserved. Terms of Use Privacy Center Accessibility Q Search LUCR + F8 F9 F10 F11 F12 * % & ( 5 6 7 8 9 Y'S Dele Insert PrtSc + Backsarrow_forward5.arrow_forward
- 9arrow_forwardalekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-IQs_pBanHhvlTCeeBZbufu BYTI0Hz7m7D3ZS18w-nDB10538ZsAtmorZoFusYj2Xu9b78gZo- O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 3- 200 temperature (K) Explanation Chick Q Sowncharrow_forward0+ aleksog/x/lsl.exe/1ou-lgNgkr7j8P3H-IQs pBaHhviTCeeBZbufuBYTOHz7m7D3ZStEPTBSB3u9bsp3Da pl19qomOXLhvWbH9wmXW5zm O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 Gab The temperature on a sample of pure X held at 0.75 atm and -229. °C is increased until the sample sublimes. The temperature is then held constant and the pressure is decreased by 0.50 atm. On the phase diagram below draw a path that shows this set of changes. F3 pressure (atm) 0- 0 200 Explanation temperature (K) Check F4 F5 ☀+ Q Search Chill Will an 9 ENG F6 F7 F8 F9 8 Delete F10 F11 F12 Insert PrtSc 114 d Ararrow_forward
- x + LEKS: Using a phase diagram a X n/alekscgi/x/lsl.exe/10_u-IgNsikr7j8P3jH-IQs_pBan HhvlTCeeBZbufu BYTI0Hz7m7D3ZcHYUt80XL-5alyVpw ○ States of Matter Using a phase diagram to find a phase transition temperature or pressure Use the phase diagram of Substance X below to find the melting point of X when the pressure above the solid is 1.1 atm. pressure (atm) 16 08- solid liquid- 0 200 400 gas 600 temperature (K) Note: your answer must be within 25 °C of the exact answer to be graded correct. × 5arrow_forwardS: Using a phase diagram leksogi/x/sl.exe/1ou-IgNs kr 7j8P3jH-IQs_pBan HhvTCeeBZbufuBYTI0Hz7m7D3ZdHYU+80XL-5alyVp O States of Matter Using a phase diagram to find a phase transition temperature or pressure se the phase diagram of Substance X below to find the boiling point of X when the pressure on the liquid is 1.6 atm. pressure (atm) 32- 16- solid liquid 0. gas 100 200 temperature (K) 300 Note: your answer must be within 12.5 °C of the exact answer to be graded correct. 10 Explanation Check § Q Search J 2025 McGraw Hill LLC. All Rights Researrow_forward151.2 254.8 85.9 199.6 241.4 87.6 242.5 186.4 155.8 257.1 242.9 253.3 256.0 216.6 108.7 239.0 149.7 236.4 152.1 222.7 148.7 278.2 268.7 234.4 262.7 283.2 143.6 QUESTION: Using this group of data on salt reduced tomato sauce concentration readings answer the following questions: 1. 95% Cl Confidence Interval (mmol/L) 2. [Na+] (mg/100 mL) 3. 95% Na+ Confidence Interval (mg/100 mL)arrow_forward
- Results Search Results Best Free Coursehero Unloc xb Success Confirmation of Q x O Google Pas alekscgi/x/lsl.exe/1o_u-IgNslkr 7j8P3jH-IQs_pBanHhvlTCeeBZbufu BYTI0Hz7m7D3ZcHYUt80XL-5alyVpwDXM TEZayFYCavJ17dZtpxbFD0Qggd1J O States of Matter Using a phase diagram to find a phase transition temperature or pressure Gabr 3/5 he pressure above a pure sample of solid Substance X at 101. °C is lowered. At what pressure will the sample sublime? Use the phase diagram of X below to nd your answer. pressure (atm) 24- 12 solid liquid gas 200 400 temperature (K) 600 ote: your answer must be within 0.15 atm of the exact answer to be graded correct. atm Thanation Check © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center I Q Search L³ ملةarrow_forward301.7 348.9 193.7 308.6 339.5 160.6 337.7 464.7 223.5 370.5 326.6 327.5 336.1 317.9 203.8 329.8 221.9 331.7 211.7 309.6 223.4 353.7 334.6 305.6 340.0 304.3 244.7 QUESTION: Using this group of data on regular tomato sauce concentration readings answer the following questions: 1. 95% Cl Confidence Interval (mmol/L) 2. [Na+] (mg/100 mL) 3. 95% Na+ Confidence Interval (mg/100 mL)arrow_forwardSearch Results Search Results Best Free Coursehero Unlo x b Success Confirmation of Q aleks.com/alekscgi/x/sl.exe/10_u-lgNslkr7j8P3jH-IQs_pBan HhvlTCeeBZbufu BYTIOHz7m7D3ZcHYUt80XL-5alyVpwDXM TEZayFYCav States of Matter Using a phase diagram to find a phase transition temperature or pressure Use the phase diagram of Substance X below to find the temperature at which X turns to a gas, if the pressure above the solid is 3.7 atm. pressure (atm) 0. 32- 16 solid liquid gas 200 temperature (K) Note: your answer must be within 20 °C of the exact answer to be graded correct. Дос Xarrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

