
Concept explainers
Indicate whether each of the following changes represents oxidation or reduction.
- a. FADH2 → FAD
- b. FMN → FMNH2
- c. Fe(III)SP → Fe(II)SP
- d. Cyt c1 (Fe3+) → cyt c1 (Fe2+)
(a)
Interpretation:
Whether the change
Concept Introduction:
Electron transport chain is a sequence of biochemical reactions in which electrons and hydrogen atoms from the citric acid cycle are transferred to various intermediate carriers and finally reacts with molecular oxygen to form a water molecule.
There are four complexes associated with the electron transport chain that is present in the inner mitochondrial membrane. The four complexes that help in the electron transfer in the electron transport chain are:
Complex I:
Complex II:
Complex III:
Complex IV:
An overview of the electron transport chain is as follows:
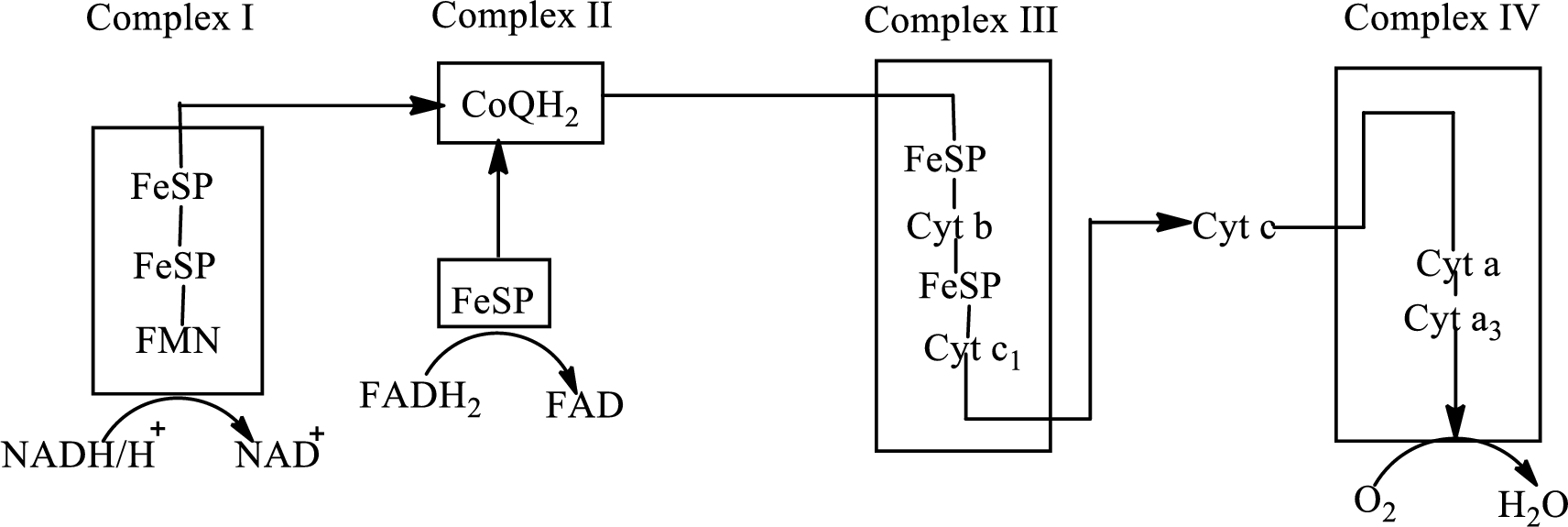
Redox reactions involve oxidation and reduction reaction occurring simultaneously so that one species is oxidized and the other one is reduced. The species that gain hydrogen or electron is known as reduced form and the species that loss hydrogen or electron is known as oxidized form. The general representation of the redox reaction is,
Here, A is oxidized form and AH is reduced form.
Answer to Problem 12.90EP
The change
Explanation of Solution
In the complex II, electrons are transferred from the
Here,
(b)
Interpretation:
Whether the change
Concept Introduction:
Electron transport chain is a sequence of biochemical reactions in which electrons and hydrogen atoms from the citric acid cycle are transferred to various intermediate carriers and finally reacts with molecular oxygen to form a water molecule.
There are four complexes associated with the electron transport chain that is present in the inner mitochondrial membrane. The four complexes that help in the electron transfer in the electron transport chain are:
Complex I:
Complex II:
Complex III:
Complex IV:
An overview of the electron transport chain is as follows:
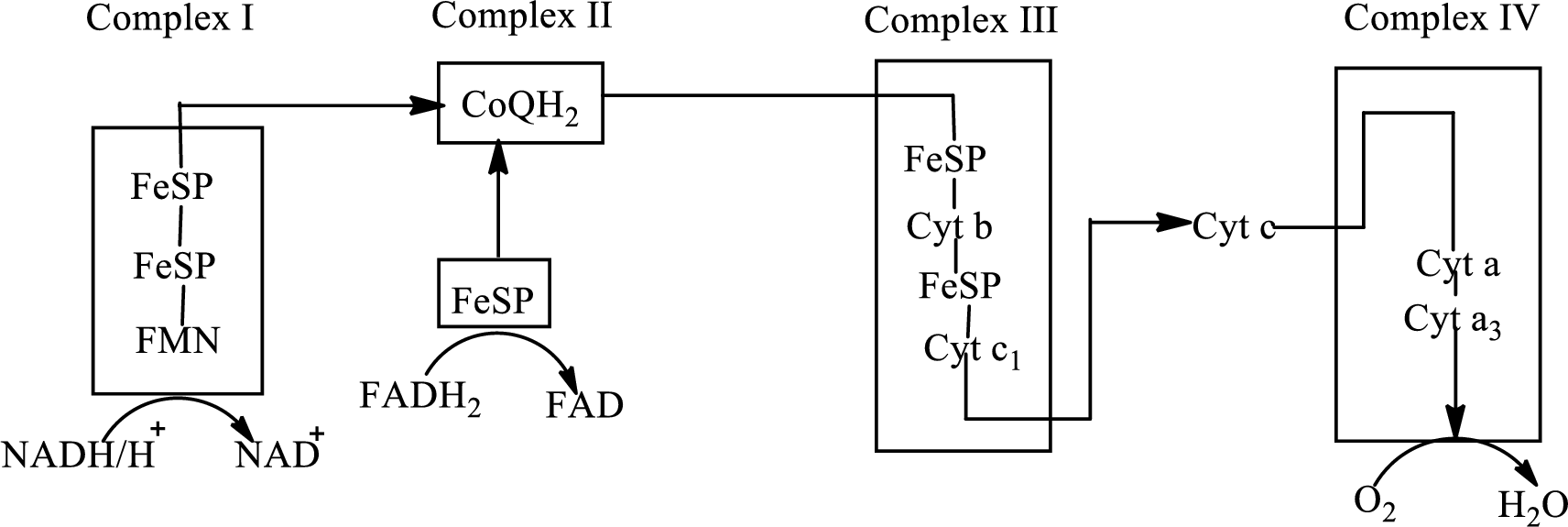
Redox reactions involve oxidation and reduction reaction occurring simultaneously so that one species is oxidized and the other one is reduced. The species that gain hydrogen or electron is known as reduced form and the species that loss hydrogen or electron is known as oxidized form. The general representation of redox reaction is,
Here, A is oxidized form and AH is reduced form.
Answer to Problem 12.90EP
The change
Explanation of Solution
Flavin mononucleotide is the structural component of the complex I of the electron transport chain. Flavin mononucleotide exists in two forms: FMN (oxidized form) and
Here, FMN gains electrons and hydrogen and leads to the formation of
(c)
Interpretation:
Whether the change
Concept Introduction:
Electron transport chain is a sequence of biochemical reactions in which electrons and hydrogen atoms from the citric acid cycle are transferred to various intermediate carriers and finally reacts with molecular oxygen to form a water molecule.
There are four complexes associated with the electron transport chain that is present in the inner mitochondrial membrane. The four complexes that help in the electron transfer in the electron transport chain are:
Complex I:
Complex II:
Complex III:
Complex IV:
An overview of the electron transport chain is as follows:
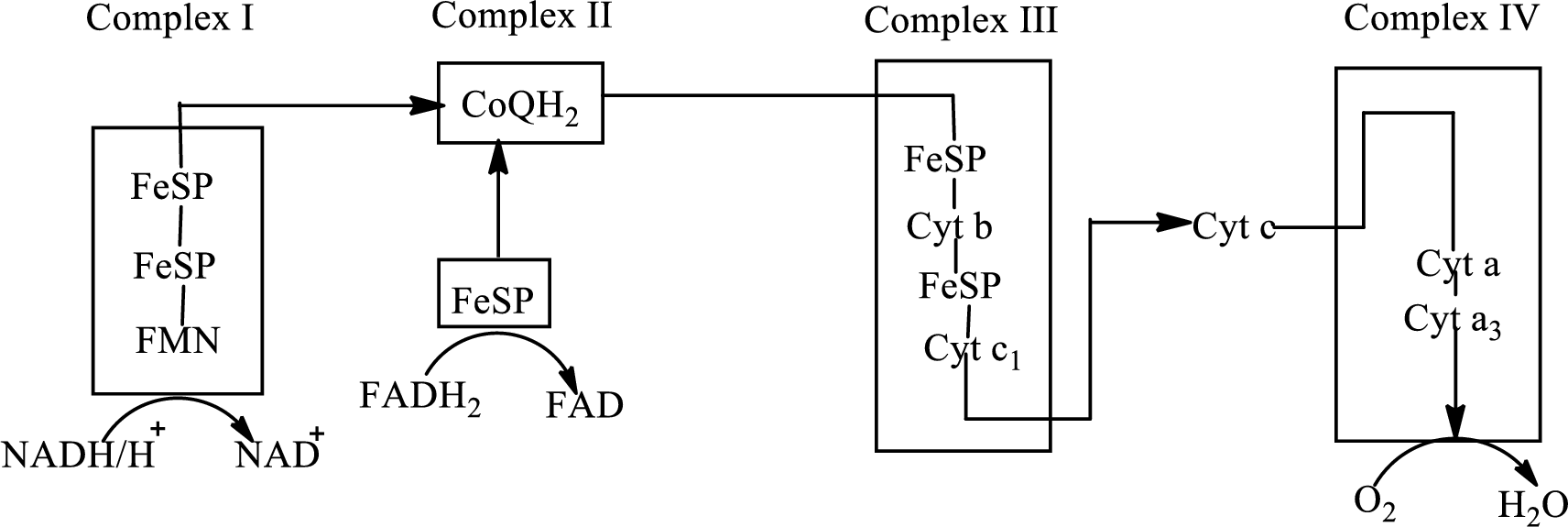
Redox reactions involve oxidation and reduction reaction occurring simultaneously so that one species is oxidized and the other one is reduced. The species that gain hydrogen or electron is known as reduced form and the species that loss hydrogen or electron is known as oxidized form. The general representation of redox reaction is,
Here, A is oxidized form and AH is reduced form.
Answer to Problem 12.90EP
The change
Explanation of Solution
Iron-sulfur proteins
(d)
Interpretation:
To indicate whether the change
Concept Introduction:
Electron transport chain is a sequence of biochemical reactions in which electrons and hydrogen atoms from the citric acid cycle are transferred to various intermediate carriers and finally reacts with molecular oxygen to form a water molecule.
There are four complexes associated with the electron transport chain that is present in the inner mitochondrial membrane. The four complexes that help in the electron transfer in the electron transport chain are:
Complex I:
Complex II:
Complex III:
Complex IV:
An overview of the electron transport chain is as follows:
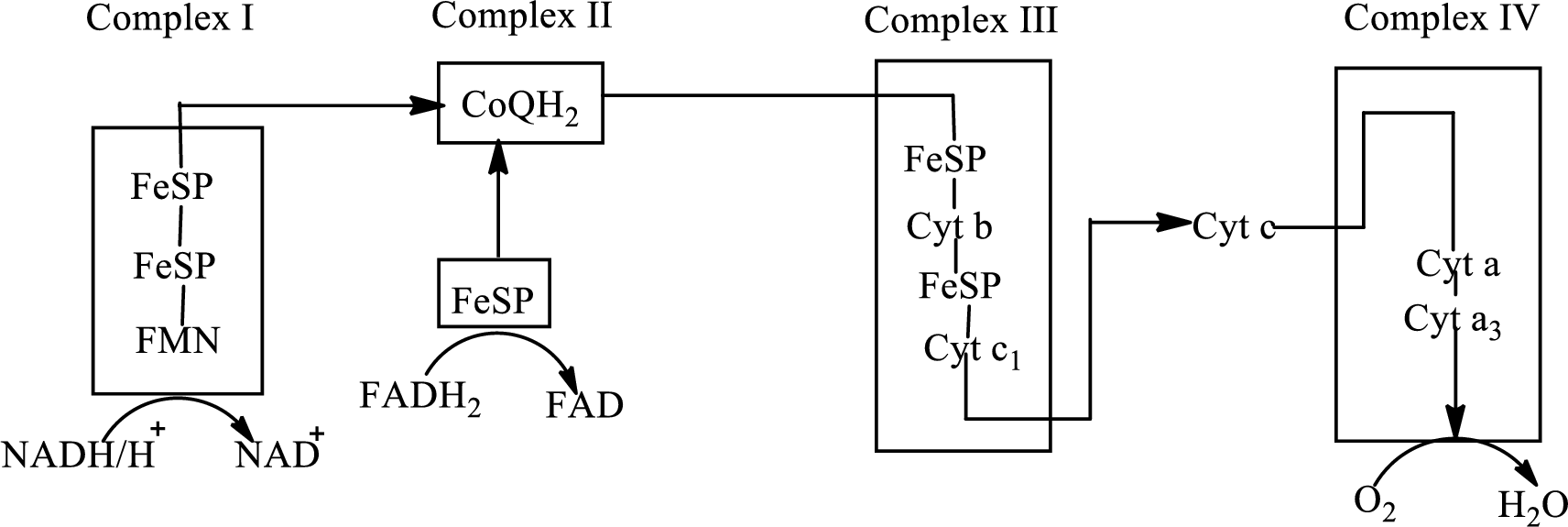
Redox reactions involve oxidation and reduction reaction occurring simultaneously so that one species is oxidized and the other one is reduced. The species that gain hydrogen or electron is known as reduced form and the species that loss hydrogen or electron is known as oxidized form. The general representation of redox reaction is,
Here, A is oxidized form and AH is reduced form.
Answer to Problem 12.90EP
The change
Explanation of Solution
Cytochromes are a structural component of the complex III and consist of iron that changes its oxidation state from
Want to see more full solutions like this?
Chapter 12 Solutions
Organic And Biological Chemistry
Additional Science Textbook Solutions
Physics for Scientists and Engineers
Human Biology: Concepts and Current Issues (8th Edition)
SEELEY'S ANATOMY+PHYSIOLOGY
General, Organic, and Biological Chemistry - 4th edition
Laboratory Manual For Human Anatomy & Physiology
- 3. Consider the compounds below and determine if they are aromatic, antiaromatic, or non-aromatic. In case of aromatic or anti-aromatic, please indicate number of I electrons in the respective systems. (Hint: 1. Not all lone pair electrons were explicitly drawn and you should be able to tell that the bonding electrons and lone pair electrons should reside in which hybridized atomic orbital 2. You should consider ring strain- flexibility and steric repulsion that facilitates adoption of aromaticity or avoidance of anti- aromaticity) H H N N: NH2 N Aromaticity (Circle) Aromatic Aromatic Aromatic Aromatic Aromatic Antiaromatic Antiaromatic Antiaromatic Antiaromatic Antiaromatic nonaromatic nonaromatic nonaromatic nonaromatic nonaromatic aromatic TT electrons Me H Me Aromaticity (Circle) Aromatic Aromatic Aromatic Aromatic Aromatic Antiaromatic Antiaromatic Antiaromatic Antiaromatic Antiaromatic nonaromatic nonaromatic nonaromatic nonaromatic nonaromatic aromatic πT electrons H HH…arrow_forwardA chemistry graduate student is studying the rate of this reaction: 2 HI (g) →H2(g) +12(g) She fills a reaction vessel with HI and measures its concentration as the reaction proceeds: time (minutes) [IH] 0 0.800M 1.0 0.301 M 2.0 0.185 M 3.0 0.134M 4.0 0.105 M Use this data to answer the following questions. Write the rate law for this reaction. rate = 0 Calculate the value of the rate constant k. k = Round your answer to 2 significant digits. Also be sure your answer has the correct unit symbol.arrow_forwardNonearrow_forward
- in which spectral range of EMR, atomic and ionic lines of metal liesarrow_forwardQ2: Label the following molecules as chiral or achiral, and label each stereocenter as R or S. CI CH3 CH3 NH2 C CH3 CH3 Br CH3 X &p Bra 'CH 3 "CH3 X Br CH3 Me - N OMe O DuckDuckarrow_forward1. For the four structures provided, Please answer the following questions in the table below. a. Please draw π molecular orbital diagram (use the polygon-and-circle method if appropriate) and fill electrons in each molecular orbital b. Please indicate the number of π electrons c. Please indicate if each molecule provided is anti-aromatic, aromatic, or non- aromatic TT MO diagram Number of π e- Aromaticity Evaluation (X choose one) Non-aromatic Aromatic Anti-aromatic || ||| + IVarrow_forward
- 1.3 grams of pottasium iodide is placed in 100 mL of o.11 mol/L lead nitrate solution. At room temperature, lead iodide has a Ksp of 4.4x10^-9. How many moles of precipitate will form?arrow_forwardQ3: Circle the molecules that are optically active: ДДДДarrow_forward6. How many peaks would be observed for each of the circled protons in the compounds below? 8 pts CH3 CH3 ΤΙ A. H3C-C-C-CH3 I (₁₁ +1)= 7 H CI B. H3C-C-CI H (3+1)=4 H LIH)=2 C. (CH3CH2-C-OH H D. CH3arrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning





