
Concept explainers
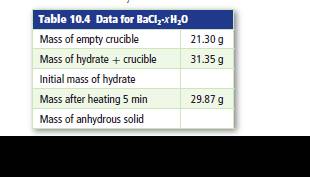
Table 4 shows data from an experiment to determine the formulas of hydrated barium chloride. Determine the formula for the hydrate and its name.
Interpretation:
The formula and name of the given hydrate of barium needs to be determined.
Concept introduction:
Number of water molecules present per molecule of a compound is known as water of crystallization.
Answer to Problem 181A
Name of the hydrate is barium chloride dihydrate.
Formula is BaCl2.2H2O.
Explanation of Solution
Molar mass of BaCl2 is 208 g/mol.
Molar mass of H2O is 18 g/mol.
Total mass of hydrated salt and crucible is 31.35 g.
Mass of the crucible is 21.30 g
Thus,
Mass of hydrated salt
Mass of the salt and crucible after 5 minutes heating is 29.87 g.
Mass of anhydrous salt
Now, number of moles of barium chloride and water can be calculated as follows:
Thus,
Moles of BaCl2
Moles of H2O
Moles ratio of BaCl2:H2O
Formula is BaCl2.2H2O.
Thus, the formula of the hydrate is BaCl2.2H2O which is called barium chloride dihydrate.
Chapter 10 Solutions
Glencoe Chemistry: Matter and Change, Student Edition
Additional Science Textbook Solutions
Brock Biology of Microorganisms (15th Edition)
Biology: Life on Earth (11th Edition)
Campbell Biology (11th Edition)
The Cosmic Perspective (8th Edition)
Introductory Chemistry (6th Edition)
Campbell Biology in Focus (2nd Edition)
- I want to know how to do it , please helparrow_forwardHelp me i dont know how to do itarrow_forwardCan you explain how to draw a molecular orbital diagram for the given molecule? It is quite difficult to understand. Additionally, could you provide a clearer illustration? Furthermore, please explain how to draw molecular orbital diagrams for any other given molecule or compound as well.arrow_forward
- Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Prob 10: Select to Add Arrows THEarrow_forwardCurved arrows are used to illustrate the flow of electrons using the provided starting and product structures draw the curved electron pushing arrows for the following reaction or mechanistic steps Ether(solvent)arrow_forwardThis deals with synthetic organic chemistry. Please fill in the blanks appropriately.arrow_forward
- Use the References to access important values if needed for this question. What is the IUPAC name of each of the the following? 0 CH3CHCNH₂ CH3 CH3CHCNHCH2CH3 CH3arrow_forwardYou have now performed a liquid-liquid extraction protocol in Experiment 4. In doing so, you manipulated and exploited the acid-base chemistry of one or more of the compounds in your mixture to facilitate their separation into different phases. The key to understanding how liquid- liquid extractions work is by knowing which layer a compound is in, and in what protonation state. The following liquid-liquid extraction is different from the one you performed in Experiment 4, but it uses the same type of logic. Your task is to show how to separate apart Compound A and Compound B. . Complete the following flowchart of a liquid-liquid extraction. Handwritten work is encouraged. • Draw by hand (neatly) only the appropriate organic compound(s) in the boxes. . Specify the reagent(s)/chemicals (name is fine) and concentration as required in Boxes 4 and 5. • Box 7a requires the solvent (name is fine). • Box 7b requires one inorganic compound. • You can neatly complete this assignment by hand and…arrow_forwardb) Elucidate compound D w) mt at 170 nd shows c-1 stretch at 550cm;' The compound has the ff electronic transitions: 0%o* and no a* 1H NMR Spectrum (CDCl3, 400 MHz) 3.5 3.0 2.5 2.0 1.5 1.0 0.5 ppm 13C{H} NMR Spectrum (CDCl3, 100 MHz) Solvent 80 70 60 50 40 30 20 10 0 ppm ppm ¹H-13C me-HSQC Spectrum ppm (CDCl3, 400 MHz) 5 ¹H-¹H COSY Spectrum (CDCl3, 400 MHz) 0.5 10 3.5 3.0 2.5 2.0 1.5 1.0 10 15 20 20 25 30 30 -35 -1.0 1.5 -2.0 -2.5 3.0 -3.5 0.5 ppm 3.5 3.0 2.5 2.0 1.5 1.0 0.5 ppmarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





