
Concept explainers
Interpretation:
The difference between an empirical formula and molecular formula should be predicted.
Concept introduction:
The representation of atoms of a compound in simple whole number ratio is known as empirical formula.
Molecular formula or Chemical formula represents the type and number of atoms present in the molecule using atomic symbol and numerical subscripts.
Answer to Problem 156A
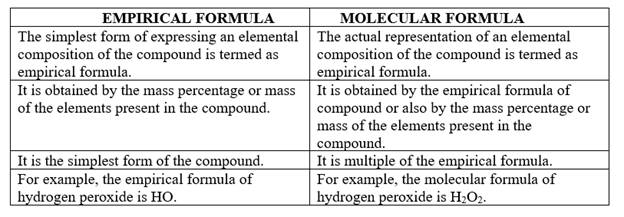
The difference between an empirical formula and molecular formula is represented as follows:
| EMPIRICAL FORMULA | MOLECULAR FORMULA |
| The simplest form of expressing an elemental composition of the compound is termed as empirical formula. | The actual |
| It is obtained by the mass percentage or mass of the elements present in the compound. | It is obtained by the empirical formula of compound or also by the mass percentage or mass of the elements present in the compound. |
| It is the simplest form of the compound. | It is multiple of the empirical formula. |
| For example, the empirical formula of hydrogen peroxide is HO. | For example, the molecular formula of hydrogen peroxide is H2O2. |
Explanation of Solution
The difference between an empirical formula and molecular formula is represented as follows:
Chapter 10 Solutions
Glencoe Chemistry: Matter and Change, Student Edition
Additional Science Textbook Solutions
Genetic Analysis: An Integrated Approach (3rd Edition)
Campbell Biology (11th Edition)
Introductory Chemistry (6th Edition)
Organic Chemistry (8th Edition)
Brock Biology of Microorganisms (15th Edition)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
- Indicate the products obtained by reacting benzenesulfonic acid with a sulfonitric acid mixture (HNO3 + H2SO4). Indicate the majority if necessary.arrow_forwardIndicate the products obtained by reacting ethylbenzene with a sulfonitric acid mixture (HNO3 + H2SO4). Indicate the majority if necessary.arrow_forwardIndicate the products obtained when tert-butylbenzene reacts with a sulfonitric acid mixture (HNO3 + H2SO4). Indicate the majority if necessary.arrow_forward
- Indicate the products obtained when acetophenone reacts with a sulfonitric acid mixture (HNO3 + H2SO4). Indicate the majority if necessary.arrow_forwardIndicate the products obtained from the reaction of N-(4-methylphenyl)acetamide with a sulfonitric acid mixture (H2SO4 + HNO3). Indicate the majority if necessary.arrow_forwardIndicate the products obtained from the reaction of 4-(trifluoromethyl)benzonitrile with a sulfonitric mixture (H2SO4 + HNO3). Indicate the majority if necessary.arrow_forward
- Indicate the products obtained in the reaction of p-Toluidine with a sulfonitric acid mixture (H2SO4 + HNO3). Indicate the majority if necessary.arrow_forwardIndicate the products obtained from the reaction of 4-methylbenzonitrile with a sulfonitric acid mixture (H2SO4 + HNO3). Indicate the majority if necessary.arrow_forwardIndicate the products obtained from the reaction of 2-nitrophenol with a sulfonitric acid mixture (H2SO4 + HNO3). Indicate the majority if necessary.arrow_forward
- In organic chemistry, what is the correct name for the mixture H2SO4 + HNO3 used in reactions: sulphonitric mixture or sulfonitric mixture?arrow_forwardFormulate the products obtained by reacting p-toluidine with a sulfonate mixture. Indicate the majority if necessary.arrow_forwardConsider this organic reaction: OH Draw the major products of the reaction in the drawing area below. If there won't be any major products, because this reaction won't happen at a significant rate, check the box under the drawing area instead. Click and drag to start drawing a structure. x 0: の Carrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





