
Draw all constitutional isomers having molecular formula C4H100. Classify each compound as a 1°, 2°, or 3° alcohol, or a symmetrical or unsymmetrical ether.
Interpretation:
To draw all the constitutional isomers for the molecular formula C4H10O and classify each compound as a 1°, 2°, or 3° alcohol, or a symmetrical or unsymmetrical ether.
Concept introduction:
Constitutional isomers also known as structural isomers. They are molecules that have the same chemical formula but their atoms are arranged differently. They are three main types of constitutional isomers.
a) Chain isomers – the carbon chain has a different order of carbon and hydrogen atoms.
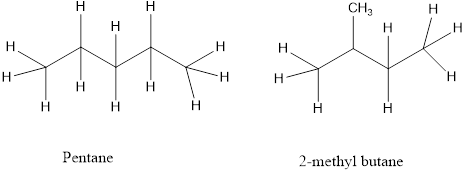
b) Position isomers- a functional group of the isomers is in a different location on the molecules.
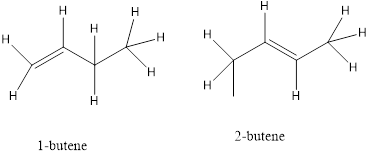
c) Functional isomers- the isomers contain a different functional group.
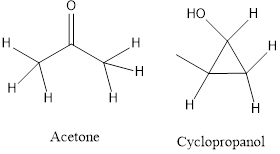
Answer to Problem 9.1P
Molecular formula C4H10O may be an alcohol and ether and seven isomers are shown below.
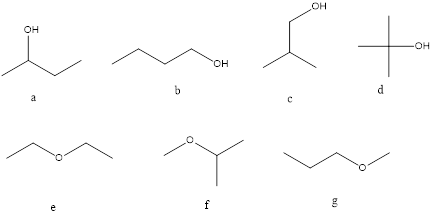
Strucutre,
A= 2o alcohol
B=1o alcohol
C=1o alcohol
D=3o alcohol
E=Symmetrical ether
F=Unsymmetrical ether
G=Unsymmetrical ether
Explanation of Solution
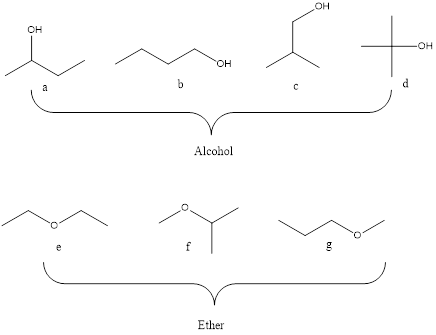
Alcohol are classified into 1o, 2o and 3o alcohol based on the number of the carbon atom bonded to carbon with OH functional group. Whereas the ether are symmetrical and unsymmetreical ethers. The ether that have two identical R groups is said to be symmetrical ether and having different R group is said to unsymmetrical ether.
Strucutre,
A= 2o alcohol
B=1o alcohol
C=1o alcohol
D=3o alcohol
E=Symmetrical ether
F=Unsymmetrical ether
G=Unsymmetrical ether
Thus seven constitutional isomers with functional groups alcohol and ether are obtained for the molecular formula C4H10O. And the structure,
A= 2o alcohol
B=1o alcohol
C=1o alcohol
D=3o alcohol
E=Symmetrical ether
F=Unsymmetrical ether
G=Unsymmetrical ether
Want to see more full solutions like this?
Chapter 9 Solutions
Organic Chemistry-Package(Custom)
- From your calculations, which reaction experiment had closest to stoichiometric quantities? How many moles of NaHCO3 and HC2H3O2 were present in this reaction?arrow_forward18. Arrange the following carbocations in order of decreasing stability. 1 2 A 3124 B 4213 C 2431 D 1234 E 2134 SPL 3 4arrow_forwardAcetic acid is added to DI water at an initial concentration of 10 -6 M (Ka=1.8x10-5) A. Using the "ICE" Method, what would the pH be at equilibrium? State assumptions and show your work. B. Using the simultaneous equations method, what would the pH be at equilibrium? Show your workarrow_forward
- 1. Show that the change in entropy for a fixed amount of ideal gas held at a constant temperature undergoing a volume change is given by the simple equation AS = NkB In Hint: Start with the equation M dS = du + (Œ) dv - Ž (#) an, dU du+av-dN; j=1 Why doesn't the equation for the entropy of an ideal gas depend on the strength of the intermolecular forces for the gas?arrow_forward2. Make an ice cube at 1 bar pressure by freezing an amount of liquid water that is 2 cm x 2 cm x 2 cm in volume. The density of liquid water at 0 °C is 1.000 g cm³ and the density of ice at 0 °C is 0.915 g cm³. Note that this difference in density is the reason your water pipes burst if they freeze and why you shouldn't forget to take your bottle of pop out of the freezer if you put it in there to try and cool it down faster. A. What is the work of expansion upon freezing? B. Is work done on the system or by the system?arrow_forwardI have a excitation/emission spectra of a quinine standard solution here, and I'm having trouble interpreting it. the red line is emission the blue line is excitation. i'm having trouble interpreting properly. just want to know if there is any evidence of raman or rayleigh peaks in the spectra.arrow_forward
- Give the major product of the following reaction. excess 1. OH, H₂O 1.OH H CH3CH2CH21 H 2. A.-H₂O Draw the molecule on the canvas by choosing buttons from the Tools (for bonds), Atoms, and Advanced Template toolbars. The single bond is active by default.arrow_forward2. Use Hess's law to calculate the AH (in kJ) for: rxn CIF(g) + F2(g) → CIF 3 (1) using the following information: 2CIF(g) + O2(g) → Cl₂O(g) + OF 2(g) AH = 167.5 kJ ΔΗ 2F2 (g) + O2(g) → 2 OF 2(g) 2C1F3 (1) + 202(g) → Cl₂O(g) + 3 OF 2(g) о = = -43.5 kJ AH = 394.1kJarrow_forwardci Draw the major product(s) of the following reactions: (3 pts) CH3 HNO3/H2SO4 HNO3/ H2SO4 OCH3 (1 pts)arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning



