
Concept explainers
Answer each question using the ball-and-stick model of compound A.
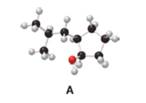
a. Give the IUPAC name for A, including
b. Classify A as a
c. Draw a stereoisomer for A and give its IUPAC name.
d. Draw a constitutional isomer that contains an
e. Draw a constitutional isomer that contains an ether and give its IUPAC name.
f. Draw the products formed (including stereochemistry) when A is treated with each reagent:
(a)
Interpretation: The IUPAC name for A, including
Concept introduction: One should follow the given steps to give the IUPAC name of cyclic alcohol. The first step is naming of ring that contains the carbon bonded to the hydroxyl group. While naming, the -e ending of parent cycloalkane must changed to the suffix -ol. The second step is numbering of carbon chain by providing lowest number to the
Answer to Problem 9.36P
The IUPAC name for A (including
Explanation of Solution
The given structure of alcohol is in the form of ball-and-stick model. It is converted into skeletal structure by replacing black ball with
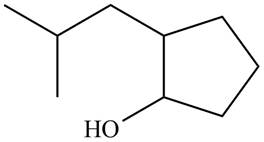
Figure 1
One should follow the given steps to give the IUPAC name of cyclic alcohol. The first step is naming of ring that contains the carbon bonded to the hydroxyl group. While naming, the -e ending of parent cycloalkane must changed to the suffix -ol. The second step is numbering of carbon chain by providing lowest number to the
The above structure of cyclic alcohol shows that the cyclic ring consists of
There are two stereogenic centers in the given structure (shown by
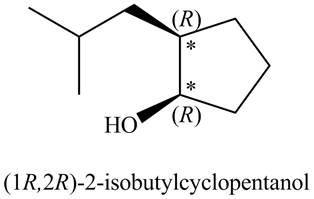
Figure 2
Thus, the IUPAC name for A (including
The IUPAC name for A (including
(b)
Interpretation: The given alcohol is to be classified as a
Concept introduction: Alcohols involve a hydroxyl group
Answer to Problem 9.36P
The given alcohol is classified as a
Explanation of Solution
Alcohols involve a hydroxyl group
In the given structure the carbon atom of alcohol (A) is bonded to two
The given alcohol is classified as a
(c)
Interpretation: A stereoisomer for ‘A’ is to be drawn, and its IUPAC name is to be stated.
Concept introduction: Isomers that differ to each other only in the way of orientation of atoms in space are called stereoisomers. They possess same functional group and same IUPAC name except for the prefixes (like cis, trans,
Answer to Problem 9.36P
A stereoisomer for ‘A’ (including its IUPAC name) is drawn in Figure 3.
Explanation of Solution
Isomers that differ to each other only in the way of orientation of atoms in space are called stereoisomers. They possess same functional group and same IUPAC name except for the prefixes (like cis, trans,
A stereoisomer for ‘A’ (including its IUPAC name) is drawn in Figure 3.
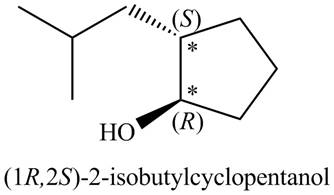
Figure 3
A stereoisomer for ‘A’ (including its IUPAC name) is drawn in Figure 3.
(d)
Interpretation: A constitutional isomer for A that contains an
Concept introduction: Isomers that differ in the way of connectivity of atoms to each other are called constitutional isomers. They may possess same or different functional groups. However, their IUPAC names are entirely different.
Answer to Problem 9.36P
A constitutional isomer for ‘A’ that contains an
Explanation of Solution
Isomers that differ in the way of connectivity of atoms to each other are called constitutional isomers. They may possess same or different functional groups. However, their IUPAC names are entirely different.
A constitutional isomer for ‘A’ that contains an
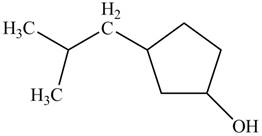
Figure 4
The above structure of cyclic alcohol shows that the cyclic ring consists of
A constitutional isomer for ‘A’ that contains an
(e)
Interpretation: A constitutional isomer for A that contains an ether is to be drawn, and its IUPAC name is to be stated.
Concept introduction: Isomers that differ in the way of connectivity of atoms to each other are called constitutional isomers. They may possess same or different functional groups. However, their IUPAC names are entirely different.
Answer to Problem 9.36P
A constitutional isomer for ‘A’ that contains an ether is drawn in Figure 5. Its IUPAC name is
Explanation of Solution
Isomers that differ in the way of connectivity of atoms to each other are called constitutional isomers. They may possess same or different functional groups. However, their IUPAC names are entirely different.
A constitutional isomer for ‘A’ that contains ether is drawn below.
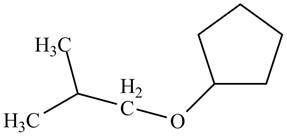
Figure 5
The above structure of cyclic alcohol shows that the cyclic ring consists of
A constitutional isomer for ‘A’ that contains an ether is drawn in Figure 5. Its IUPAC name is
(f)
Interpretation: The products (including stereochemistry) formed from the treatment of A with the each reagent are to be stated.
Concept introduction: In proton transfer reactions, the stereochemistry of the product is retained. The
Answer to Problem 9.36P
The products formed from the treatment of A with the reagent
Explanation of Solution
Products formed from reagent
The given reagent is
An alkoxide salt is required to prepare ether. The alkoxide salts are prepared from alcohols through the Bronsted-Lowry acid-base reaction. In this reaction,
The reaction involves only transfer of proton. Since the reaction does not involve cleavage of the
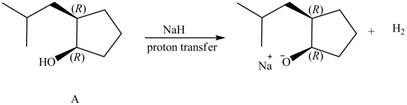
Figure 6
Products formed from reagent
The given reagent is
Alcohols undergo dehydration reaction in the presence of strong acids like
Thus, the products formed from the treatment of A with the given reagent are,
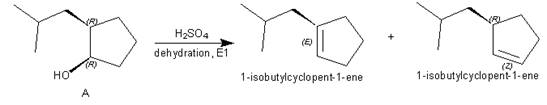
Figure 7
Mixture of products is obtained due to carbocation rearrangement, and having two
Products formed from reagent
The given reagent is
Alcohols undergo dehydration reaction in the presence of
Thus, the products formed from the treatment of A with the given reagent are,
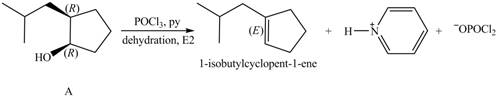
Figure 8
Products formed from reagent
The given reagent is
The reaction of alcohols with halogen acids
Since the given alcohol, A is
Thus, the products formed from the treatment of A with the given reagent are,
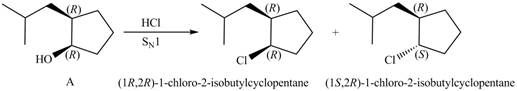
Figure 9
Products formed from reagent
The given reagent is
Alkyl chlorides are obtained by the reaction of
Since the given alcohol, A is
Thus, the products formed from the treatment of A with the given reagent are,
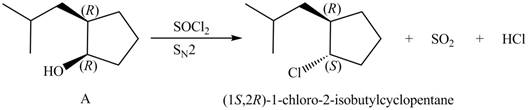
Figure 10
Products formed from reagent
The given reagent is
Alcohols are converted into alkyl tosylates by treatment with
Thus, the products formed from the treatment of A with the given reagent are,
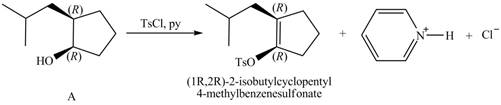
Figure 11
The products formed from the treatment of A with the reagent
Want to see more full solutions like this?
Chapter 9 Solutions
Organic Chemistry-Package(Custom)
Additional Science Textbook Solutions
Biology: Life on Earth with Physiology (11th Edition)
Applications and Investigations in Earth Science (9th Edition)
Biology: Life on Earth (11th Edition)
Fundamentals Of Thermodynamics
Campbell Essential Biology with Physiology (5th Edition)
Human Biology: Concepts and Current Issues (8th Edition)
- Are there any alternative methods better than the MOHR titration to quantitatively determine salt in a sample?arrow_forwardhybridization of nitrogen of complex moleculesarrow_forwardUsing reaction free energy to predict equilibrium composition Consider the following equilibrium: 2NO2 (g) = N2O4(g) AGº = -5.4 kJ Now suppose a reaction vessel is filled with 4.53 atm of dinitrogen tetroxide (N2O4) at 279. °C. Answer the following questions about this system: Under these conditions, will the pressure of N2O4 tend to rise or fall? Is it possible to reverse this tendency by adding NO2? In other words, if you said the pressure of N2O4 will tend to rise, can that be changed to a tendency to fall by adding NO2? Similarly, if you said the pressure of N2O4 will tend to fall, can that be changed to a tendency to '2' rise by adding NO2? If you said the tendency can be reversed in the second question, calculate the minimum pressure of NO 2 needed to reverse it. Round your answer to 2 significant digits. 00 rise ☐ x10 fall yes no ☐ atm G Ar 1arrow_forward
- Why do we analyse salt?arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. H H CH3OH, H+ H Select to Add Arrows H° 0:0 'H + Q HH ■ Select to Add Arrows CH3OH, H* H. H CH3OH, H+ HH ■ Select to Add Arrows i Please select a drawing or reagent from the question areaarrow_forwardWhat are examples of analytical methods that can be used to analyse salt in tomato sauce?arrow_forward
- A common alkene starting material is shown below. Predict the major product for each reaction. Use a dash or wedge bond to indicate the relative stereochemistry of substituents on asymmetric centers, where applicable. Ignore any inorganic byproducts H Šali OH H OH Select to Edit Select to Draw 1. BH3-THF 1. Hg(OAc)2, H2O =U= 2. H2O2, NaOH 2. NaBH4, NaOH + Please select a drawing or reagent from the question areaarrow_forwardWhat is the MOHR titration & AOAC method? What is it and how does it work? How can it be used to quantify salt in a sample?arrow_forwardPredict the major products of this reaction. Cl₂ hv ? Draw only the major product or products in the drawing area below. If there's more than one major product, you can draw them in any arrangement you like. Be sure you use wedge and dash bonds if necessary, for example to distinguish between major products with different stereochemistry. If there will be no products because there will be no significant reaction, just check the box under the drawing area and leave it blank. Note for advanced students: you can ignore any products of repeated addition. Explanation Check Click and drag to start drawing a structure. 80 10 m 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility DII A F1 F2 F3 F4 F5 F6 F7 F8 EO F11arrow_forward
- Given a system with an anodic overpotential, the variation of η as a function of current density- at low fields is linear.- at higher fields, it follows Tafel's law.Calculate the range of current densities for which the overpotential has the same value when calculated for both cases (the maximum relative difference will be 5%, compared to the behavior for higher fields).arrow_forwardUsing reaction free energy to predict equilibrium composition Consider the following equilibrium: N2 (g) + 3H2 (g) = 2NH3 (g) AGº = -34. KJ Now suppose a reaction vessel is filled with 8.06 atm of nitrogen (N2) and 2.58 atm of ammonia (NH3) at 106. °C. Answer the following questions about this system: rise Under these conditions, will the pressure of N2 tend to rise or fall? ☐ x10 fall Is it possible to reverse this tendency by adding H₂? In other words, if you said the pressure of N2 will tend to rise, can that be changed to a tendency to fall by adding H2? Similarly, if you said the pressure of N will tend to fall, can that be changed to a tendency to rise by adding H₂? If you said the tendency can be reversed in the second question, calculate the minimum pressure of H₂ needed to reverse it. Round your answer to 2 significant digits. yes no ☐ atm Х ด ? olo 18 Ararrow_forwardFour liters of an aqueous solution containing 6.98 mg of acetic acid were prepared. At 25°C, the measured conductivity was 5.89x10-3 mS cm-1. Calculate the degree of dissociation of the acid and its ionization constant.Molecular weights: O (15.999), C (12.011), H (1.008).Limiting molar ionic conductivities (λ+0 and λ-0) of Ac-(aq) and H+(aq): 40.9 and 349.8 S cm-2 mol-1.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





