
Batch processes are often used in chemical and pharmaceutical operations to achieve a desired chemicalcomposition for the final product. Related heat transferprocesses are typically transient, involving a liquid a fixed volume that may be heated from room temperature to a desired process temperature, or cooled fromthe process temperature to room temperature. Considera batch process for which a pharmaceutical (the coldfluid. c) ¡s poured into an insulated, highly agitated vessel (a stirred reactor) and heated by passing a hot fluid(h) through a submerged heat exchanger coil of thin-walled tubing and surface area
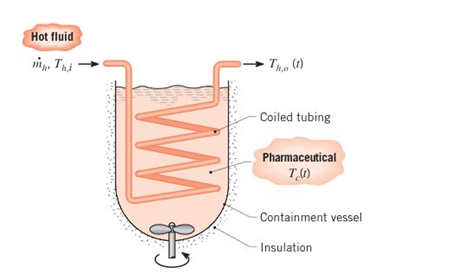
(a) Starting from basic principles, derive expressions thatcan be used to determine the variation of
(b) Consider a pharmaceutical of volume
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
Fundamentals of Heat and Mass Transfer
Additional Engineering Textbook Solutions
Vector Mechanics For Engineers
Database Concepts (8th Edition)
Introduction To Programming Using Visual Basic (11th Edition)
Starting Out with Python (4th Edition)
Thermodynamics: An Engineering Approach
Management Information Systems: Managing The Digital Firm (16th Edition)
- Can you solve it analytically using laplace transforms and with Matlab code as well please. Thank You.arrow_forwardQ11. Determine the magnitude of the reaction force at C. 1.5 m a) 4 KN D b) 6.5 kN c) 8 kN d) e) 11.3 KN 20 kN -1.5 m- C 4 kN -1.5 m B Mechanical engineering, No Chatgpt.arrow_forwardplease help with this practice problem(not a graded assignment, this is a practice exam), and please explain how to use sohcahtoaarrow_forward
- Solve this problem and show all of the workarrow_forwardSolve this problem and show all of the workarrow_forwardaversity of Baoyion aculty of Engineering-AIMusyab Automobile Eng. Dep. Year: 2022-2023, st Course, 1st Attempt Stage: 3rd Subject: Heat Transfer I Date: 2023\01\23- Monday Time: 3 Hours Q4: A thick slab of copper initially at a uniform temperature of 20°C is suddenly exposed to radiation at one surface such that the net heat flux is maintained at a constant value of 3×105 W/m². Using the explicit finite-difference techniques with a space increment of Ax = = 75 mm, determine the temperature at the irradiated surface and at an interior point that is 150 mm from the surface after 2 min have elapsed. Q5: (12.5 M) A) A steel bar 2.5 cm square and 7.5 cm long is initially at a temperature of 250°C. It is immersed in a tank of oil maintained at 30°C. The heat-transfer coefficient is 570 W/m². C. Calculate the temperature in the center of the bar after 3 min. B) Air at 90°C and atmospheric pressure flows over a horizontal flat plate at 60 m/s. The plate is 60 cm square and is maintained at a…arrow_forward
- University of Baby on Faculty of Engineering-AIMusyab Automobile Eng. Dep. Year: 2022-2023. 1 Course, 1" Attempt Stage 3 Subject Heat Transfer I Date: 2023 01 23- Monday Time: 3 Hours Notes: Q1: • • Answer four questions only Use Troles and Appendices A) A flat wall is exposed to an environmental temperature of 38°C. The wall is covered with a layer of insulation 2.5 cm thick whose thermal conductivity is 1.4 W/m. C, and the temperature of the wall on the inside of the insulation is 315°C. The wall loses heat to the environment by convection. Compute the value of the convection heat-transfer coefficient that must be maintained on the outer surface of the insulation to ensure that the outer-surface temperature does not exceed 41°C. B) A vertical square plate, 30 cm on a side, is maintained at 50°C and exposed to room air at 20°C. The surface emissivity is 0.8. Calculate the total heat lost by both sides of the plate. (12.5 M) Q2: An aluminum fin 1.5 mm thick is placed on a circular tube…arrow_forwardSolve using graphical method and analytical method, only expert should solvearrow_forwardSolve this and show all of the workarrow_forward
 Principles of Heat Transfer (Activate Learning wi...Mechanical EngineeringISBN:9781305387102Author:Kreith, Frank; Manglik, Raj M.Publisher:Cengage Learning
Principles of Heat Transfer (Activate Learning wi...Mechanical EngineeringISBN:9781305387102Author:Kreith, Frank; Manglik, Raj M.Publisher:Cengage Learning
