
Concept explainers
(a)
Interpretation:
Molecular shape, bond angle and hybrid orbital of
Concept introduction:
Lewis structure is also called as Lewis dot structure. In this structure, valence electrons are represented by dot symbol around the central atom. This structure shows the bonding between molecule and the lone pairs which may occur in the molecule.
Answer to Problem 112A
Molecular shape of
Explanation of Solution
Valence electron of Cl is 7 and S is 6. Total number of valence electrons in
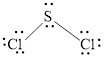
Central atom contains total 4 pair of electrons, two non bonding pairs and 2 bonding electron pairs. The molecular shape of
(b)
Interpretation:
Molecular shape, bond angle and hybrid orbital of
Concept introduction:
Lewis structure is also called as Lewis dot structure. In this structure, valence electrons are represented by dot symbol around the central atom. This structure shows the bonding between molecule and the lone pairs which may occur in the molecule.
Answer to Problem 112A
Molecular shape of
Explanation of Solution
Valence electron of both N is 5, Cl is 7 and H is 1. Total number of valance electrons in
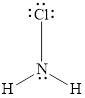
Central atom contains total 4 pair of electrons, one non bonding pair and 3 bonding electron pairs. The molecular shape of
(c)
Interpretation:
Molecular shape, bond angle and hybrid orbital of
Concept introduction:
Lewis structure is also called as Lewis dot structure. In this structure, valence electrons are represented by dot symbol around the central atom. This structure shows the bonding between molecule and the lone pairs which may occur in the molecule.
Answer to Problem 112A
Molecular shape of
Explanation of Solution
Valence electron of both O is 6, F is 7 and H is 1. Total number of valence electrons in
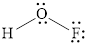
Central atom contains total 4 pair of electrons, two non bonding pair and 2 bonding electron pairs. The molecular shape of
(d)
Interpretation:
Molecular shape, bond angle and hybrid orbital of
Concept introduction:
Lewis structure is also called as Lewis dot structure. In this structure, valence electrons are represented by dot symbol around the central atom. This structure shows the bonding between molecule and the lone pairs which may occur in the molecule.
Answer to Problem 112A
Molecular shape of
Explanation of Solution
Valance electron of B is 3 and F is 7. Hence, total number of valance electron is
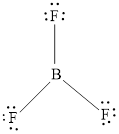
Central atom contains total 3 bonding electron pairs. The molecular shape of
Chapter 8 Solutions
Glencoe Chemistry: Matter and Change, Student Edition
Additional Science Textbook Solutions
Chemistry: A Molecular Approach (4th Edition)
Biology: Life on Earth (11th Edition)
Campbell Biology in Focus (2nd Edition)
Campbell Essential Biology (7th Edition)
Chemistry: Structure and Properties (2nd Edition)
Introductory Chemistry (6th Edition)
- 5. Count the electrons on the following complexes and state whether they follow the 18- electron rule: (3 points) Fe(CO)5 Ni(PMe3)4 PMe3 is trimethylphosphine Mn(CO)5Brarrow_forwardFor questions 1-4, consider the following complexes: [Co(CN)6]+, [CoCl4]², [Cr(H2O)6]²+ 2. Draw the corresponding d-orbital splitting for each of the complexes; predict the spin- state (low-spin/high spin) for each of the complexes (if applicable); explain your arguments. Calculate the crystal field stabilization energy for each complex (in Ao or At). (6 points)arrow_forwardFor questions 1-4, consider the following complexes: [Co(CN)6]4, [COC14]², [Cr(H2O)6]²+ 1. Assign oxidation number to the metal, then indicate d-electron count. (3 points)arrow_forward
- Using iodometry I want to titrate a sodium thiosulfate solution and I use 15 mL. If I have 50 mL of a 0.90 M copper solution and KI, what will be the molarity of sodium thiosulfate?arrow_forwardDraw the product formed when the following pair of compounds is treated with NaOEt in ethanol. + i CNarrow_forwardI need help with the followingarrow_forward
- I need help with the followingarrow_forwardFor Raman spectroscopy/imaging, which statement is not true regarding its disadvantages? a) Limited spatial resolution. b) Short integration time. c) A one-dimensional technique. d) Weak signal, only 1 in 108 incident photons is Raman scattered. e) Fluorescence interference.arrow_forwardUsing a cell of known pathlength b = 1.25115 x 10-3 cm, a water absorption spectrum was measured. The band at 1645 cm-1, assigned to the O-H bending, showed an absorbance, A, of 1.40. a) Assuming that water density is 1.00 g/mL, calculate the water molar concentration c (hint: M= mole/L) b) Calculate the molar absorptivity, a, of the 1645 cm-1 band c) The transmitted light, I, can be written as I= Ioexp(-xb), where x is the absorption coefficient (sometimes designated as alpha), Io is the input light, and b is the cell pathlength. Prove that x= (ln10)*x*c. (Please provide a full derivation of the equation for x from the equation for I). d) Calculate x for the 1645 cm-1 bandarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





