
Concept explainers
Interpretation:
Resonance structure of
Concept introduction:
A Resonance structure is the two or more correct Lewis structures which indicate a single molecule or ion. These structures differ only in electron pairs position. The location of atom is same in resonance structure.
Answer to Problem 103A
Two resonance structure of
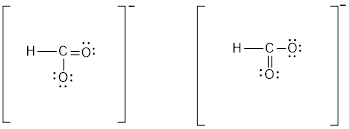
Explanation of Solution
Resonance is a condition for those molecules which have more than one valid Lewis structure. The
have two possible Lewis structure. In these structure only position of double bond and electron pairs are different. Valence electron of O is 6, C is four, hydrogen is 1 and one more electron due to negative charge. Total number of valence electron is 18. To complete octet, C forms a double bond with any of one oxygen atoms. Hence, the resonance structure is as follows:
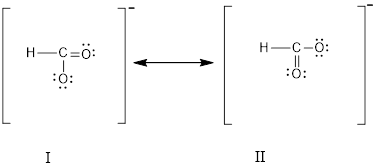
The above two structure represents
Each molecule or ion that undergoes resonance behaves as if it has only one structure.
Chapter 8 Solutions
Glencoe Chemistry: Matter and Change, Student Edition
Additional Science Textbook Solutions
Microbiology: An Introduction
Organic Chemistry (8th Edition)
Campbell Biology: Concepts & Connections (9th Edition)
Campbell Essential Biology (7th Edition)
Introductory Chemistry (6th Edition)
Genetic Analysis: An Integrated Approach (3rd Edition)
- Please see photoarrow_forward=Naming benzene derivatives Name these organic compounds: structure C1 CH3 name ☐ CH3 ப C1 × ☐arrow_forwardBlocking Group are use to put 2 large sterically repulsive group ortho. Show the correct sequence toconnect the reagent to product with the highest yield possible. * see image **NOTE: The compound on the left is the starting point, and the compound on the right is the final product. Please show the steps in between to get from start to final, please. These are not two different compounds that need to be worked.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





