
Concept explainers
Interpretation:
The chemical composition of a detergent is to be stated. The explanation as to how detergent removes oil and grease from fabric is to be stated.
Concept introduction:
The process via which soaps and detergents remove the dust particles (dust, grease, oil, etc) from the cloths or any other surface is termed as a cleaning process.
Answer to Problem 142A
The chemical composition of detergent is builders, surfactants, bleaches, enzymes, and other ingredients like optical brighteners, softeners, colorants, etc. The polar part of the detergent attracts with water and the non-polar part attracts with dust particles. After scrubbing, dust particles pull away from the clothes.
Explanation of Solution
Commonly the laundry detergent is used to wash clothes. The laundry detergent is composed of the following chemicals:
1. Surfactants: Surfactants reduce the surface tension of water so that water can wet the cloth very well. Surfactant absorbs into dust particles, forms an emulsion with water to clean dust. Therefore, surfactants are accountable for cleaning. Alkylbenzene sulfonates are one of the most common surfactant.
2. Builders: It is known that some metal (Ca and Mg) carbonate and bicarbonate present in water as a mineral. These metal ions can react with surfactant scum which makes the detergent less effective. Builders act as a chelating agent that removes these metal (Ca and Mg) carbonate and bicarbonate minerals via chelation and precipitation. Zeolites, polycarboxylates, silicates, citrates, etc are the most common builders.
3. Bleaches: As the name indicates bleach is used to remove color dust. Common bleach is sodium perborate, sodium percarbonate, etc. These bleaches are inactive in solid-state but become active when contact with water.
4. Other ingredients: The other ingredients are optical brighteners, softeners, colorants, etc. Perfume is also used in today’s detergent.
Cleaning Action of Detergents: The detergent dissociates into cations and anions. For example, the ionization of detergent sodium alkyl sulfate is shown below.
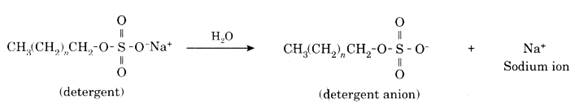
The alkyl part of the detergent anion is hydrophobic in nature which is attracted by dust particles. The sulfate part of the detergent anion is hydrophilic in nature which is attracted by water. Therefore, the detergent anion forms micelle with water. After scrubbing, dust particles pull away from the clothes.
The chemical composition of detergent is builders, surfactants, bleaches, enzymes, and other ingredients like optical brighteners, softeners, colorants, etc. The polar part of the detergent attracts with water and the nonpolar part attracts with dust particles. After scrubbing, dust particles pull away from the clothes.
Chapter 8 Solutions
Glencoe Chemistry: Matter and Change, Student Edition
Additional Science Textbook Solutions
Campbell Biology: Concepts & Connections (9th Edition)
Introductory Chemistry (6th Edition)
Human Anatomy & Physiology (2nd Edition)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Biology: Life on Earth (11th Edition)
Human Physiology: An Integrated Approach (8th Edition)
- Indicate the names of these compounds (if they exist). 0: HỌC—NH CH3CH2-CH2arrow_forwardN Classify each of the following molecules as aromatic, antiaromatic, or nonaromatic. NH O aromatic O antiaromatic O nonaromatic O aromatic O antiaromatic O nonaromatic O aromatic O antiaromatic O nonaromatic Garrow_forwardThe conjugate base of alkanes is called alkides. Correct?.arrow_forward
- Name these organic compounds: structure Br name CH3 CH3 ☐ ☐arrow_forwardHH H-C H -C-H HH Draw the Skeletal Structures & H Name the molecules HH H H H H-C-C-C-C-C-C-H HHH HHH H H HHHHHHH H-C-C-C-C-C-C-C-C-C-H HHHHH H H H Harrow_forwarddont provide AI solution .... otherwise i will give you dislikearrow_forward
- Name these organic compounds: structure name CH3 CH3 ☐ F F CH3 ☐ O Explanation Check 2025 McGraw Hill LLC. All Rights Reserved. Terms ofarrow_forwardClassify each of the following molecules as aromatic, antiaromatic, or nonaromatic. ZI NH Explanation Check O aromatic O antiaromatic O nonaromatic O aromatic O antiaromatic H O nonaromatic O aromatic O antiaromatic O nonaromatic ×arrow_forwardPart I. Draw the stepwise reaction mechanism of each product (a, b, c, d, e, f) HO HO OH НОН,С HO OH Sucrose HO CH₂OH H N N HO -H H -OH KMnO4, Heat H OH CH₂OH (d) Phenyl Osatriazole OH НОН,С HO HO + Glacial HOAC HO- HO CH₂OH OH HO Fructose (a) Glucose OH (b) H₂N HN (c) CuSO4-5H2O, ethanol H N N N HO ·H H OH H OH N CH₂OH OH (f) Phenyl Osazone H (e) Carboxy phenyl osatriazole Figure 2.1. Reaction Scheme for the Total Synthesis of Fine Chemicalsarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





