
Interpretation: The contrast between the structure of ionic compounds and metals.
Concept introduction:
The packing of molecules or atoms is usually at its lattice through networks wherein there is an interaction between the molecules. Based on the strength of interaction, the bonds are further classified as ionic, hydrogen bonding, metallic bonding and covalent bonding.
Answer to Problem 40SSC
The main difference in structure is that metals are having metal ions immersed in a sea of electrons, whereas ionic compounds are those having positive and negative charges occupying lattices
Explanation of Solution
The bonding in metals is formed when the electrons in the metal leave its outer shell to form a positively charged ion. These ions are then amidst a ‘sea’ of delocalized electrons. The structure is such that metals consist of metals ions arranged at lattice points in a regular pattern.
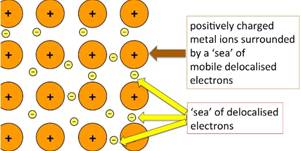
The bonding in ionic compounds is merely electrostatic forces of attraction between the positively charged ion and negatively charged ion. The metal atom will lose electron(s) to form a positive ion while a nonmetal atom will gain electron(s) to form a negative ion. Both are held by coulombic forces to give a brittle structure.
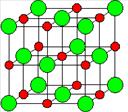
The oppositely charged ions are next to each other forming a regular pattern.
Difference in structure
| Metals | Ionic compounds |
| The positively charged ions occupy lattice points which are surrounded by a ‘sea’ of electrons. | The positively charged ions are situated next to negatively charged ion and are arranged in a regular pattern. |
| The electrons are ‘delocalized’ over the entire lattice and are free to move about. | The electrons are not delocalized and there are no free electrons. |
| The forces of attraction are electrostatic forces between a metal ion and delocalized electrons. | The forces of attraction are electrostatic forces between two oppositely charged ions. |
The main difference in structure is that metals are having metal ions immersed in a sea of electrons whereas ionic compounds are those having positive and negative charges occupying lattices.
Chapter 7 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Concepts of Genetics (12th Edition)
Laboratory Experiments in Microbiology (12th Edition) (What's New in Microbiology)
Organic Chemistry (8th Edition)
Cosmic Perspective Fundamentals
Campbell Biology: Concepts & Connections (9th Edition)
Biology: Life on Earth (11th Edition)
- If compound A reacts with an excess of methyl iodide and then heated with aqueous Ag₂O, indicate only the major products obtained. Draw their formulas. A Harrow_forwardExplanation Check 1:01AM Done 110 Functional Groups Identifying and drawing hemiacetals and acetals In the drawing area below, create a hemiacetal with 1 ethoxy group, 1 propoxy group, and a total of 9 carbon atoms. Click and drag to start drawing a structure. ✓ $ 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Sarrow_forwardWrite the systematic name of each organic molecule: CI structure CI CI Explanation CI ठ CI Check B ☐ 188 F1 80 name F2 F3 F4 F5 F6 60 F7 2arrow_forward
- Write the systematic name of each organic molecule: structure i HO OH Explanation Check name ☐ ☐arrow_forwardX 5 Check the box under each molecule that has a total of five ẞ hydrogens. If none of the molecules fit this description, check the box underneath the table. CI Br Br Br 0 None of these molecules have a total of five ẞ hydrogens. Explanation Check esc F1 F2 tab caps lock fn Q @2 A W # 3 OH O OH HO © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility IK F7 F7 F8 TA F9 F10 & 6 28 * ( > 7 8 9 0 80 F3 O F4 KKO F5 F6 S 64 $ D % 25 R T Y U பட F G H O J K L Z X C V B N M H control option command P H F11 F12 + || { [ command optionarrow_forwardAn open vessel containing water stands in a laboratory measuring 5.0 m x 5.0 m x 3.0 m at 25 °C ; the vapor pressure (vp) of water at this temperature is 3.2 kPa. When the system has come to equilibrium, what mass of water will be found in the air if there is no ventilation? Repeat the calculation for open vessels containing benzene (vp = 13.1 kPa) and mercury (vp = 0.23 Pa)arrow_forward
- Every chemist knows to ‘add acid to water with constant stirring’ when diluting a concentrated acid in order to keep the solution from spewing boiling acid all over the place. Explain how this one fact is enough to prove that strong acids and water do not form ideal solutions.arrow_forwardThe predominant components of our atmosphere are N₂, O₂, and Ar in the following mole fractions: χN2 = 0.780, χO2 = 0.21, χAr = 0.01. Assuming that these molecules act as ideal gases, calculate ΔGmix, ΔSmix, and ΔHmix when the total pressure is 1 bar and the temperature is 300 K.arrow_forwarddG = Vdp - SdT + μA dnA + μB dnB + ... so that under constant pressure and temperature conditions, the chemical potential of a component is the rate of change of the Gibbs energy of the system with respect to changing composition, μJ = (∂G / ∂nJ)p,T,n' Using first principles prove that under conditions of constant volume and temperature, the chemical potential is a measure of the partial molar Helmholtz energy (μJ = (∂A / ∂nJ)V,T,n')arrow_forward
- The vapor pressure of dichloromethane at 20.0 °C is 58.0 kPa and its enthalpy of vaporization is 32.7 kJ/mol. Estimate the temperature at which its vapor pressure is 66.0 kPa.arrow_forwardDraw the structure of A, the minor E1 product of the reaction. Cl Skip Part Check F1 esc CH_CH OH, D 3 2 Click and drag to start drawing a structure. 80 R3 F4 F2 F3 @ 2 # $ 4 3 Q W 95 % KO 5 F6 A F7 × G ☐ Save For Later Sub 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy C ►II A A F8 F9 F10 FL 6 7 88 & * 8 9 LLI E R T Y U A S D lock LL F G H 0 P J K L Z X C V B N M 9 Harrow_forwardFrom the choices given, which two substances have the same crystal structure? (Select both) Group of answer choices ZnS (zincblende) Diamond TiO2 (rutile) ZnS (wurtzite)arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





