
(a)
Interpretation:
The structure of organic product and formulas of the inorganic product formed in the given reaction has to be drawn.
Concept Introduction:
Alkylation reaction is a reaction in which the transfer of alkyl group from one molecule to another molecule takes place. While considering
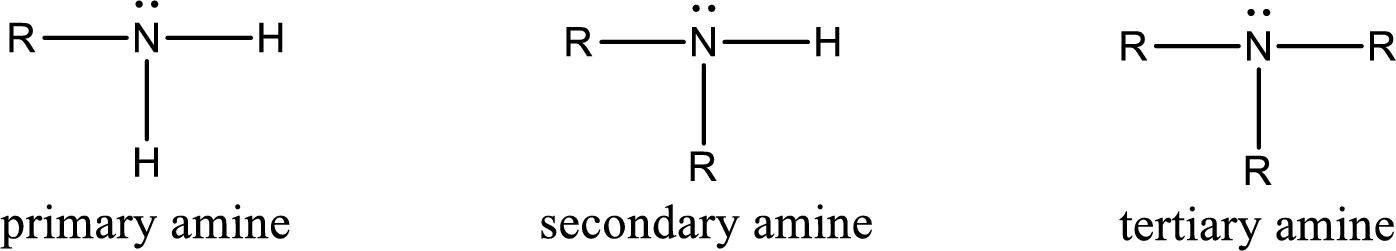
Amine is an organic derivative. If in ammonia one or more alkyl, cycloalkyl, or aryl groups are substituted instead of hydrogen atom then it is known as amine. Depending on the number of substitution the amines are classified as primary, secondary or tertiary amine. Primary amine is the one in which only one hydrogen atom in ammonia is replaced by a hydrocarbon group. Secondary amine is the one in which only two hydrogen atoms in ammonia is replaced by a hydrocarbon group. Tertiary amine is the one in which all three hydrogen atoms in ammonia is replaced by a hydrocarbon group. The generalized structural formula for all the amines is,
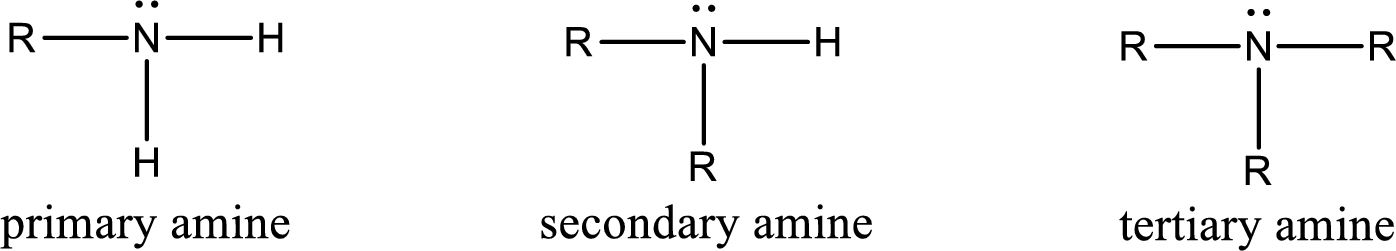
Quaternary ammonium salt is the one that has four carbon atoms attached to the nitrogen atom. This is formed by the reaction of tertiary amine with alkyl halide in presence of a strong base.
(a)
Explanation of Solution
Given reaction is,

The reactants given in the above reaction are ammonia, propyl chloride. Sodium hydroxide is a reagent that is used for basic condition in this case. As the reaction between ammonia and propyl chloride gives propylamine as the product, this is an alkylation reaction. The complete reaction can be given as,
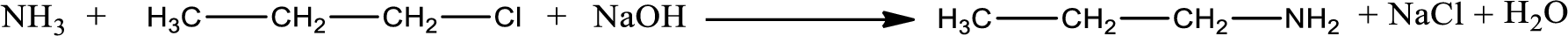
The organic product formed is propylamine. The inorganic product is sodium chloride and water molecule. The structures are shown above.
The structure of organic product and formulas of inorganic products are drawn.
(b)
Interpretation:
The structure of organic product and formulas of the inorganic product formed in the given reaction has to be drawn.
Concept Introduction:
Alkylation reaction is a reaction in which the transfer of alkyl group from one molecule to another molecule takes place. While considering amines, the alkylating agent that is used is alkyl halides. Alkylation is done under basic conditions. The general equations for amines alkylation process is,
Amine is an organic derivative. If in ammonia one or more alkyl, cycloalkyl, or aryl groups are substituted instead of hydrogen atom then it is known as amine. Depending on the number of substitution the amines are classified as primary, secondary or tertiary amine. Primary amine is the one in which only one hydrogen atom in ammonia is replaced by a hydrocarbon group. Secondary amine is the one in which only two hydrogen atoms in ammonia is replaced by a hydrocarbon group. Tertiary amine is the one in which all three hydrogen atoms in ammonia is replaced by a hydrocarbon group. The generalized structural formula for all the amines is,
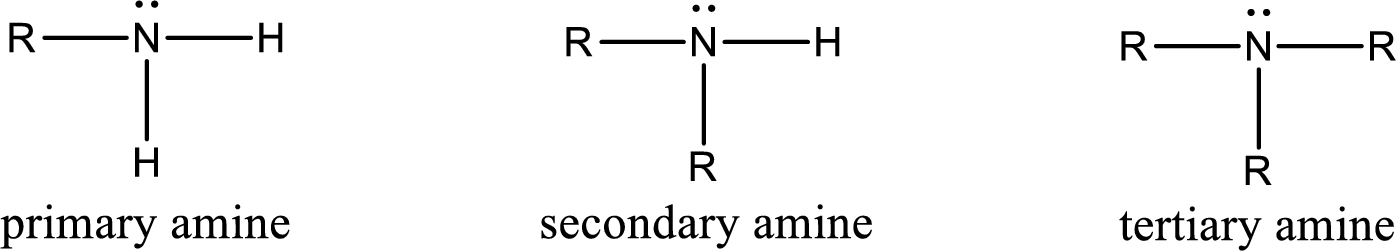
Quaternary ammonium salt is the one that has four carbon atoms attached to the nitrogen atom. This is formed by the reaction of tertiary amine with alkyl halide in presence of a strong base.
(b)
Explanation of Solution
Given reaction is,
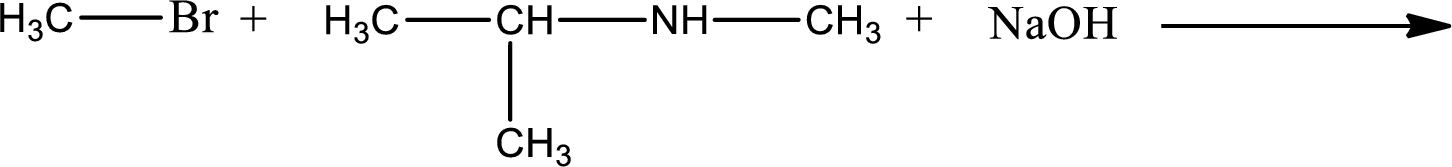
The reactants given in the above reaction are isopropylmethylamine, methyl bromide. Sodium hydroxide is a reagent that is used for basic condition in this case. As the reaction between isopropylmethylamine and methyl bromide gives isopropyldimethylamine as the product, this is an alkylation reaction. The complete reaction can be given as,
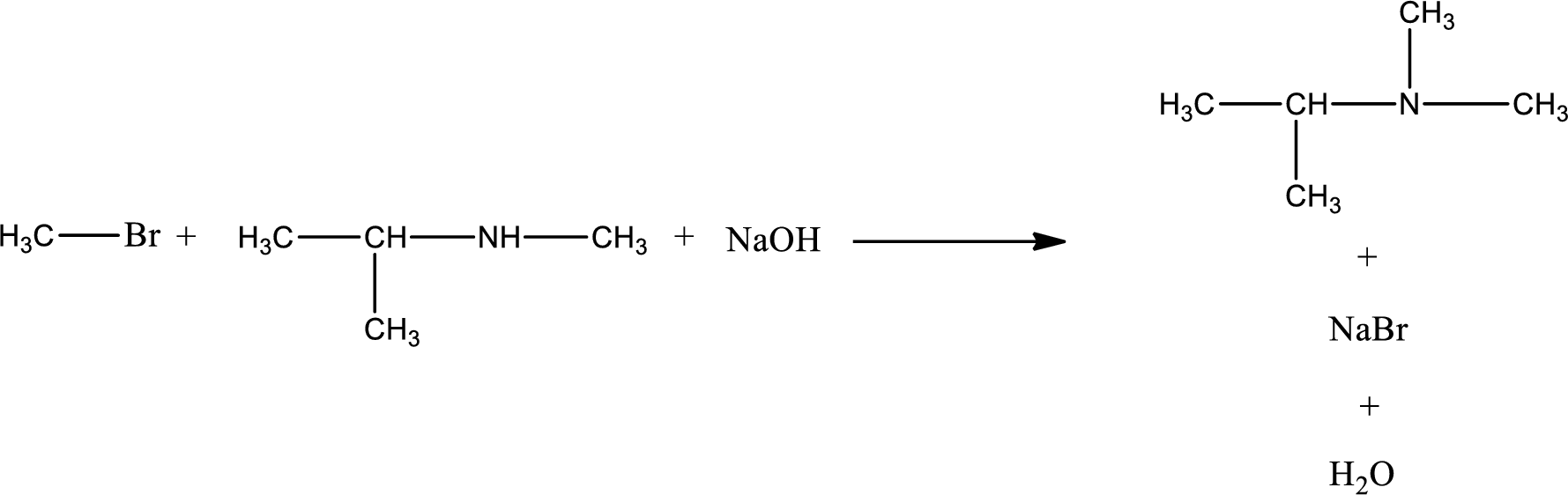
The organic product that is formed has a nitrogen atom that is bonded to three carbon atoms. The inorganic product is sodium bromide and water molecule. The structures are shown above.
The structure of organic product and formulas of inorganic products are drawn.
(c)
Interpretation:
The structure of organic product and formulas of the inorganic product formed in the given reaction has to be drawn.
Concept Introduction:
Alkylation reaction is a reaction in which the transfer of alkyl group from one molecule to another molecule takes place. While considering amines, the alkylating agent that is used is alkyl halides. Alkylation is done under basic conditions. The general equations for amines alkylation process is,
Amine is an organic derivative. If in ammonia one or more alkyl, cycloalkyl, or aryl groups are substituted instead of hydrogen atom then it is known as amine. Depending on the number of substitution the amines are classified as primary, secondary or tertiary amine. Primary amine is the one in which only one hydrogen atom in ammonia is replaced by a hydrocarbon group. Secondary amine is the one in which only two hydrogen atoms in ammonia is replaced by a hydrocarbon group. Tertiary amine is the one in which all three hydrogen atoms in ammonia is replaced by a hydrocarbon group. The generalized structural formula for all the amines is,
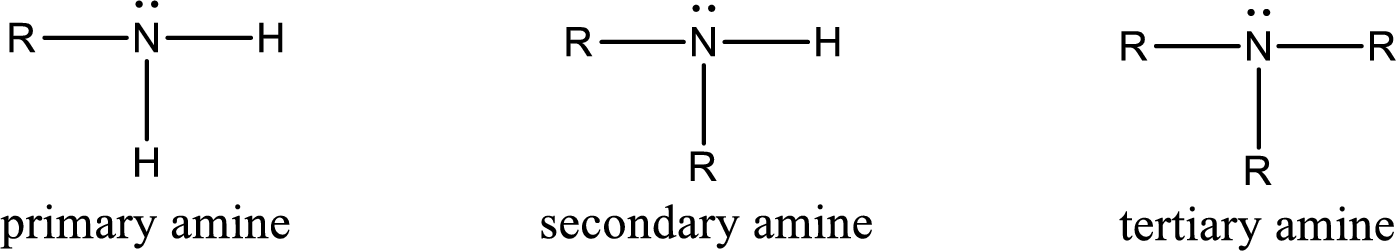
Quaternary ammonium salt is the one that has four carbon atoms attached to the nitrogen atom. This is formed by the reaction of tertiary amine with alkyl halide in presence of a strong base.
(c)
Explanation of Solution
Given reaction is,
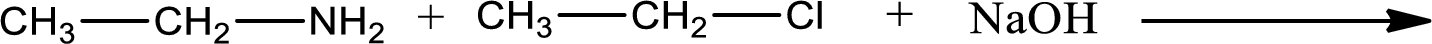
The reactants given in the above reaction are ethylamine, ethyl chloride. Sodium hydroxide is a reagent that is used for basic condition in this case. As the reaction between ethylamine and ethyl chloride gives diethylamine as the product, this is an alkylation reaction. The complete reaction can be given as,
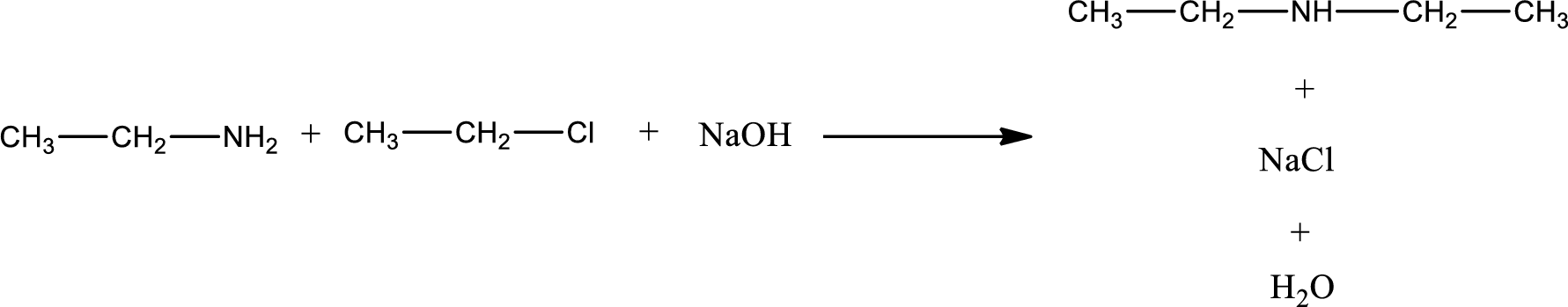
The organic product obtained has a nitrogen atom that is bonded to two carbon atoms and one hydrogen atom. The inorganic product is sodium chloride and water molecule. The structures are shown above.
The structure of organic product and formulas of inorganic products are drawn.
(d)
Interpretation:
The structure of organic product and formulas of the inorganic product formed in the given reaction has to be drawn.
Concept Introduction:
Alkylation reaction is a reaction in which the transfer of alkyl group from one molecule to another molecule takes place. While considering amines, the alkylating agent that is used is alkyl halides. Alkylation is done under basic conditions. The general equations for amines alkylation process is,
Amine is an organic derivative. If in ammonia one or more alkyl, cycloalkyl, or aryl groups are substituted instead of hydrogen atom then it is known as amine. Depending on the number of substitution the amines are classified as primary, secondary or tertiary amine. Primary amine is the one in which only one hydrogen atom in ammonia is replaced by a hydrocarbon group. Secondary amine is the one in which only two hydrogen atoms in ammonia is replaced by a hydrocarbon group. Tertiary amine is the one in which all three hydrogen atoms in ammonia is replaced by a hydrocarbon group. The generalized structural formula for all the amines is,
Quaternary ammonium salt is the one that has four carbon atoms attached to the nitrogen atom. This is formed by the reaction of tertiary amine with alkyl halide in presence of a strong base.
(d)
Explanation of Solution
Given reaction is,
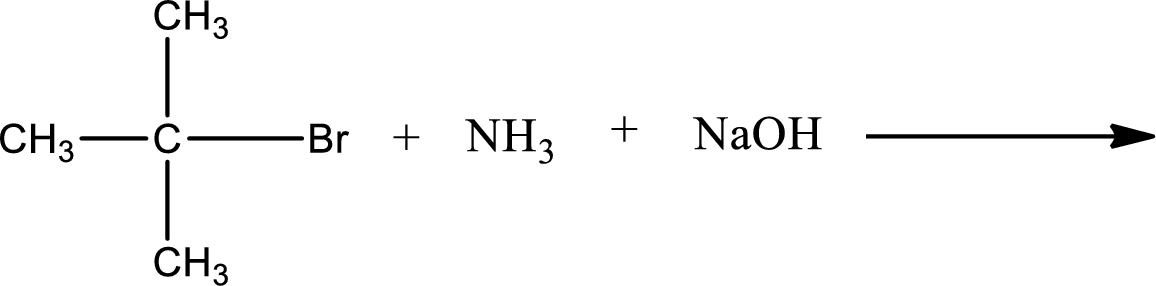
The reactants given in the above reaction are ammonia, tert-butyl bromide. Sodium hydroxide is a reagent that is used for basic condition in this case. As the reaction between ammonia and tert-butyl bromide gives tert-butylamine as the product, this is an alkylation reaction. The complete reaction can be shown as,
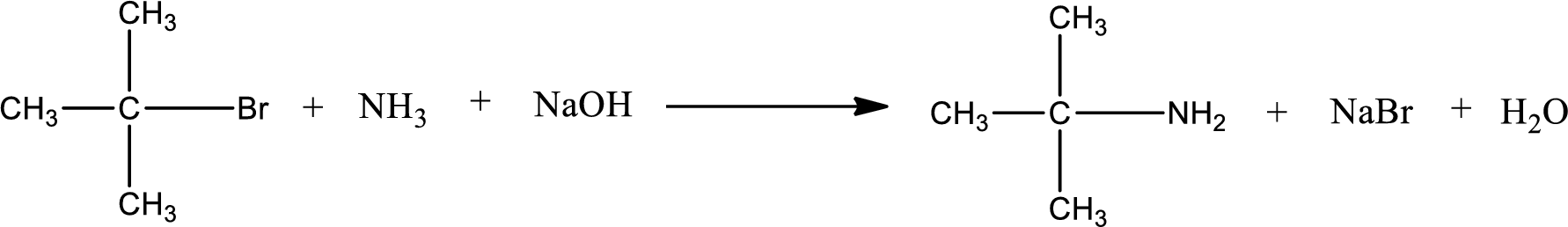
The organic product formed has a nitrogen atom that is bonded to one carbon atom and two hydrogen atoms. The inorganic product is sodium bromide and water molecule. The structures are shown above.
The structure of organic product and formulas of inorganic products are drawn.
Want to see more full solutions like this?
Chapter 6 Solutions
Organic And Biological Chemistry
- The temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forward
- er your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward5.arrow_forward6.arrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





