
Concept explainers
(a)
Interpretation:
Structure of
Concept Introduction:
Amides are synthesized using amidification reaction. This involves a reaction between  group present in carboxylic acid and
group present in carboxylic acid and 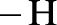 from ammonia or amine is lost to give amide as the product. Water is obtained as a by-product in this reaction. The general reaction scheme for synthesis of amides can be given as,
from ammonia or amine is lost to give amide as the product. Water is obtained as a by-product in this reaction. The general reaction scheme for synthesis of amides can be given as,
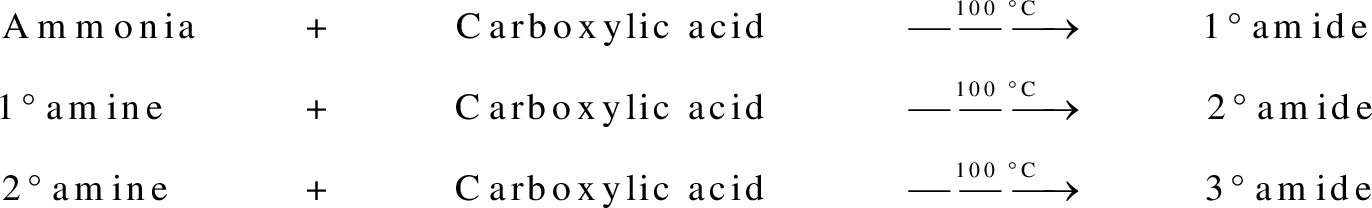
(b)
Interpretation:
Structure of carboxylic acid that is required to produce the given compound as product through amidification has to be given.
Concept Introduction:
Amides are synthesized using amidification reaction. This involves a reaction between amine and carboxylic acid. In this reaction, the  group present in carboxylic acid and
group present in carboxylic acid and 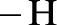 from ammonia or amine is lost to give amide as the product. Water is obtained as a by-product in this reaction. The general reaction scheme for synthesis of amides can be given as,
from ammonia or amine is lost to give amide as the product. Water is obtained as a by-product in this reaction. The general reaction scheme for synthesis of amides can be given as,
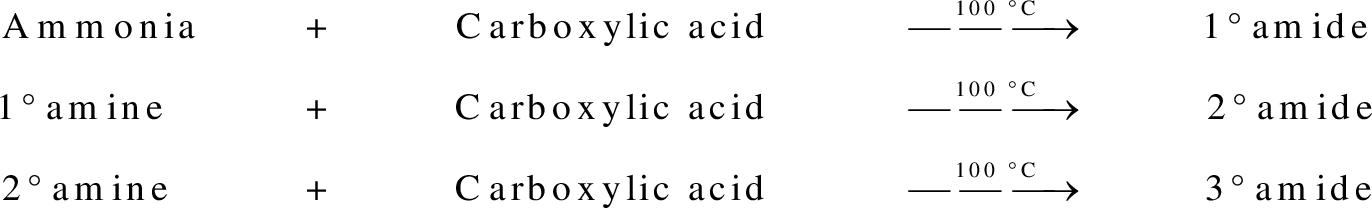
(c)
Interpretation:
Structure of carboxylic acid that is required to produce the given compound as product through amidification has to be given.
Concept Introduction:
Amides are synthesized using amidification reaction. This involves a reaction between amine and carboxylic acid. In this reaction, the  group present in carboxylic acid and
group present in carboxylic acid and 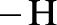 from ammonia or amine is lost to give amide as the product. Water is obtained as a by-product in this reaction. The general reaction scheme for synthesis of amides can be given as,
from ammonia or amine is lost to give amide as the product. Water is obtained as a by-product in this reaction. The general reaction scheme for synthesis of amides can be given as,
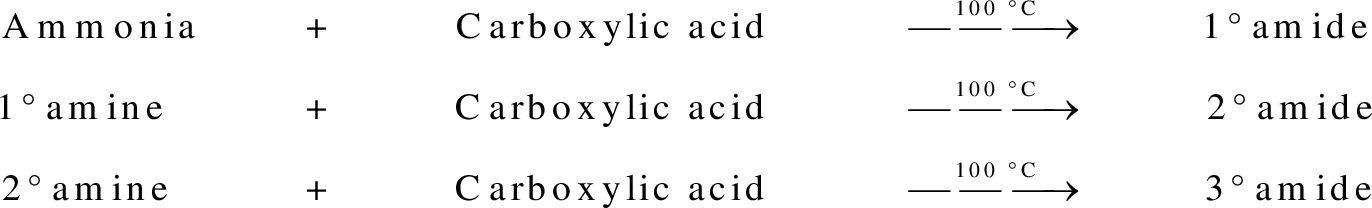
(d)
Interpretation:
Structure of carboxylic acid that is required to produce the given compound as product through amidification has to be given.
Concept Introduction:
Amides are synthesized using amidification reaction. This involves a reaction between amine and carboxylic acid. In this reaction, the  group present in carboxylic acid and
group present in carboxylic acid and 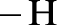 from ammonia or amine is lost to give amide as the product. Water is obtained as a by-product in this reaction. The general reaction scheme for synthesis of amides can be given as,
from ammonia or amine is lost to give amide as the product. Water is obtained as a by-product in this reaction. The general reaction scheme for synthesis of amides can be given as,
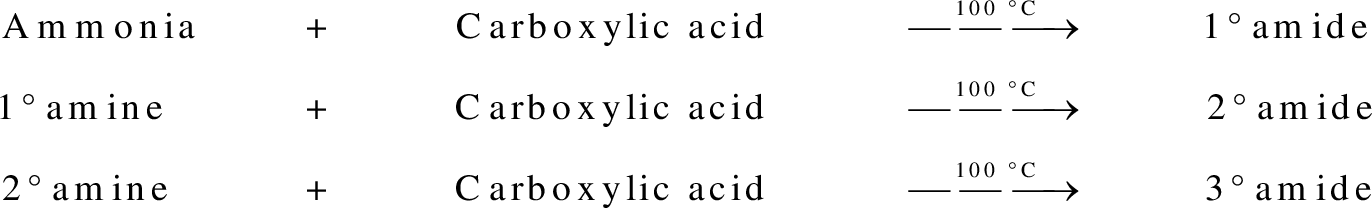
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
Organic And Biological Chemistry
- no Ai walkthroughsarrow_forward136 PRACTICAL SPECTROSCOPY Compound 78 is a high-boiling liquid (boiling point 189° C) that contains halogen, but will not react with alkoxides to yield an halogen. ether. The Mass, IR, and 'H NMR spectra, along with 13C NMR data, are given below. Elemental Analysis: C, 35.32; H, 2.47; contains BC Spectral Data: doublet, 137.4 ppm; doublet, 130.1 ppm; doublet, 127.4 ppm; singlet, 97.3 ppm Absorbance Mass Spectrum Intensity 77 77 204 M + 128 40 60 80 100 120 140 160 180 m/e 200 220 280 240 260 300 Infrared Spectrum Wave Number, cm -1 4000 3000 2500 2000 1500 1300 1200 1100 1000 900 800 700 3 6 7 8 9 10 12 13 15 Wavelength, microns 'H NMR wwwww 5 Structure: www ppm, & ©2000 Brooks/Cole Publishing Com-arrow_forwardno Ai walkthroughsarrow_forward
- 3. Synthesize the following synthon from the indicated starting material. i HO.arrow_forwardIdentifying the stereochemistry of natural Write the complete common (not IUPAC) name of each molecule below. Note: if a molecule is one of a pair of enantiomers, be sure you start its name with D- or L- so we know which enantiomer it is. molecule H O-C-CH2 H3N. HN N H C=O common name (not the IUPAC name) NH3 ☐ H3N H ☐ CH2 Xarrow_forward> Draw the structure of alanine at pH 1.2. Click and drag to start drawing a structure.arrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning



