
Concept explainers
(a)
Interpretation:
Structure of missing substance in the given reaction that involves amides has to be drawn.
Concept Introduction:
Amides are synthesized using amidification reaction. This involves a reaction between
(a)
Answer to Problem 6.138EP
Structure of the missing substance is,
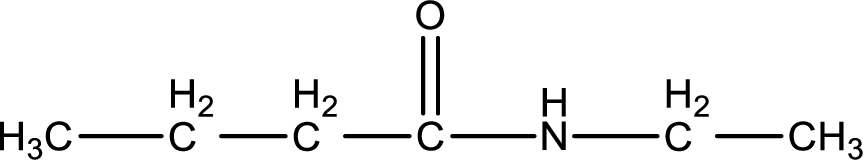
Explanation of Solution
Given reaction is,

The starting material is a carboxylic acid and a primary amine. The product obtained on amidification reaction between carboxylic acid and a primary amine is a secondary amide. This is formed by the loss of water molecule. The structure of the secondary amide that is formed can be given as,
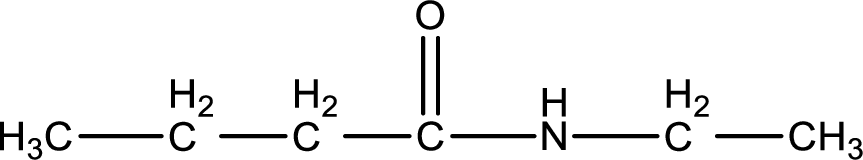
The complete reaction can be given as,
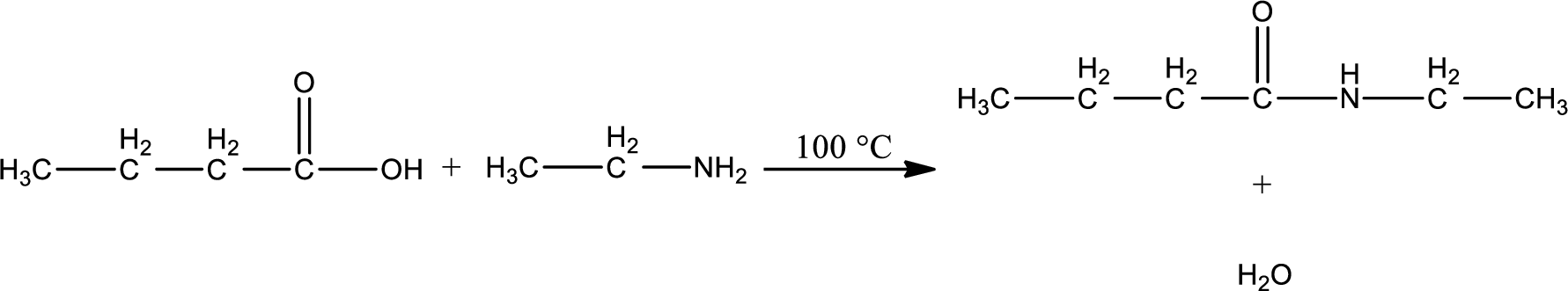
Structure of the missing compound is drawn.
(b)
Interpretation:
Structure of missing substance in the given reaction that involves amides has to be drawn.
Concept Introduction:
Amides are synthesized using amidification reaction. This involves a reaction between amine and carboxylic acid. In this reaction, the
(b)
Answer to Problem 6.138EP
Structure of the missing substance is,
Explanation of Solution
Given reaction is,
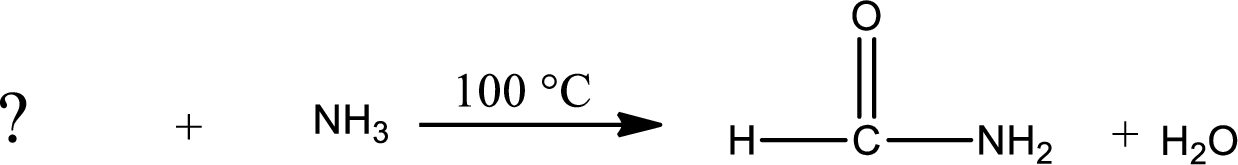
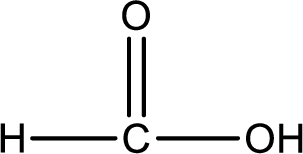
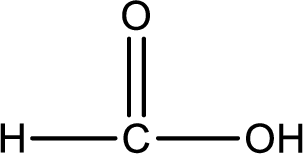
As the nitrogen atom present in the above amide has two hydrogen atoms bonded to it and the amide is a primary amide. Primary amide is produced by the reaction of ammonia with carboxylic acid. The structure of carboxylic acid can be found as shown below,
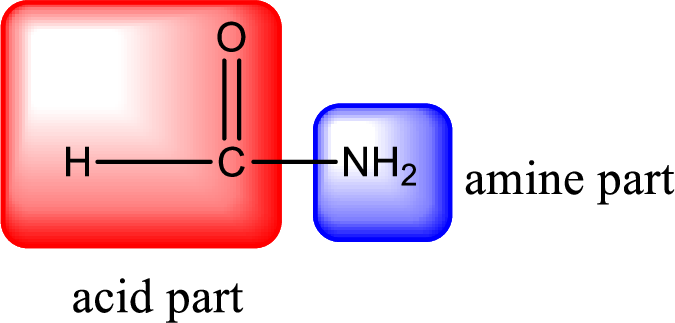
Hydrogen atom has to be added to the amine part and
The complete reaction can be given as,
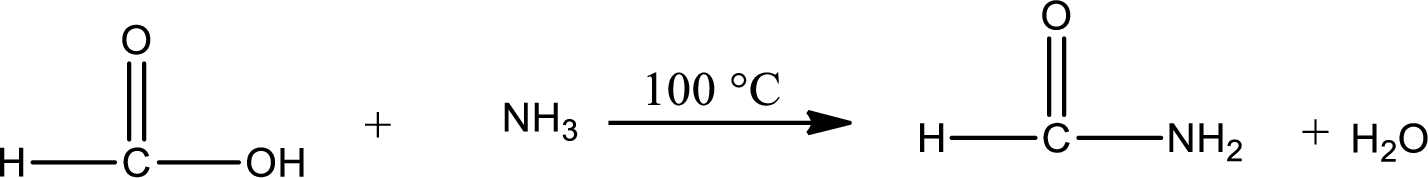
Structure of the missing compound is drawn.
(c)
Interpretation:
Structure of missing substance in the given reaction that involves amides has to be drawn.
Concept Introduction:
Amides are synthesized using amidification reaction. This involves a reaction between amine and carboxylic acid. In this reaction, the
(c)
Answer to Problem 6.138EP
Structure of the missing substance is,
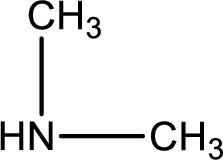
Explanation of Solution
Given reaction is,

As the nitrogen atom present in the above amide has no hydrogen atoms bonded to it, the amide is a tertiary amide. Tertiary amide is produced by the reaction of secondary amine with carboxylic acid. The parent compound structures can be identified as shown below,
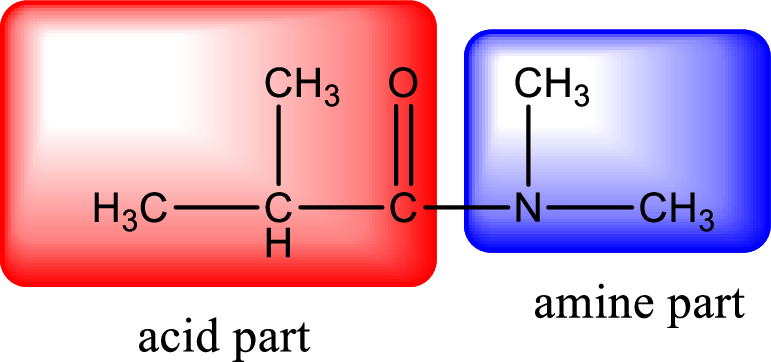
Hydrogen atom has to be added to the amine part and
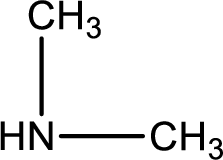
The complete reaction can be given as,

Structure of the missing compound is drawn.
(d)
Interpretation:
Structure of missing substance in the given reaction that involves amides has to be drawn.
Concept Introduction:
Amides are synthesized using amidification reaction. This involves a reaction between amine and carboxylic acid. In this reaction, the
(d)
Answer to Problem 6.138EP
Structure of the missing substance is,
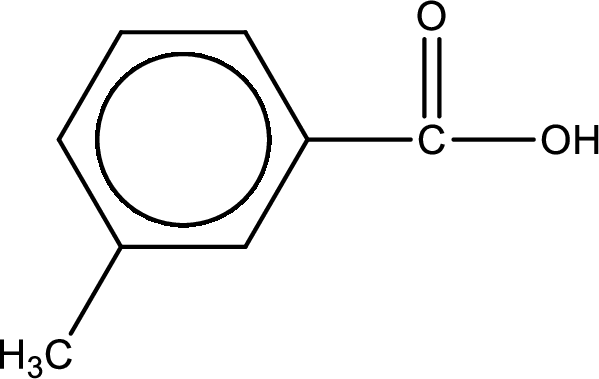
Explanation of Solution
Given reaction is,
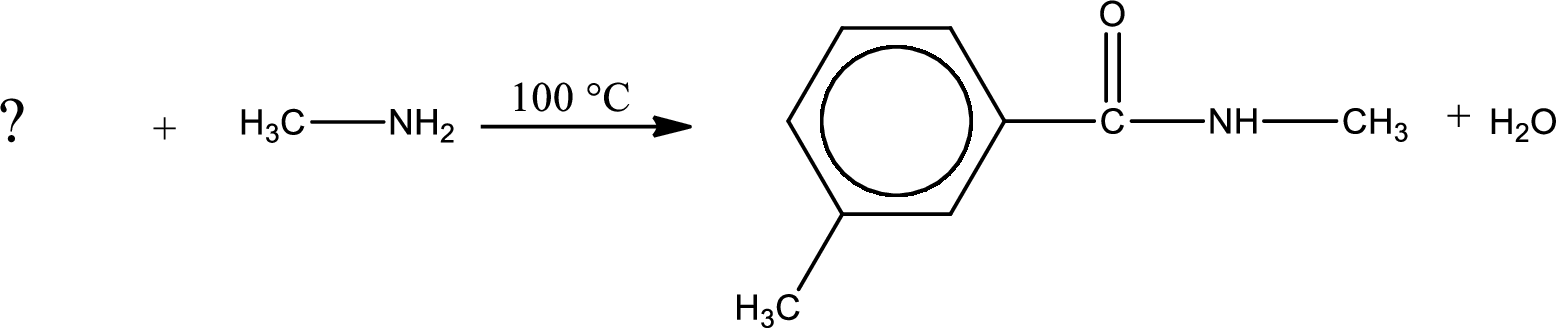
As the nitrogen atom present in the above amide has one hydrogen atom bonded to it and the amide is a secondary amide. Secondary amide is produced by the reaction of primary amine with carboxylic acid. The structure of carboxylic acid is given and the structure of primary amine has to be found out.
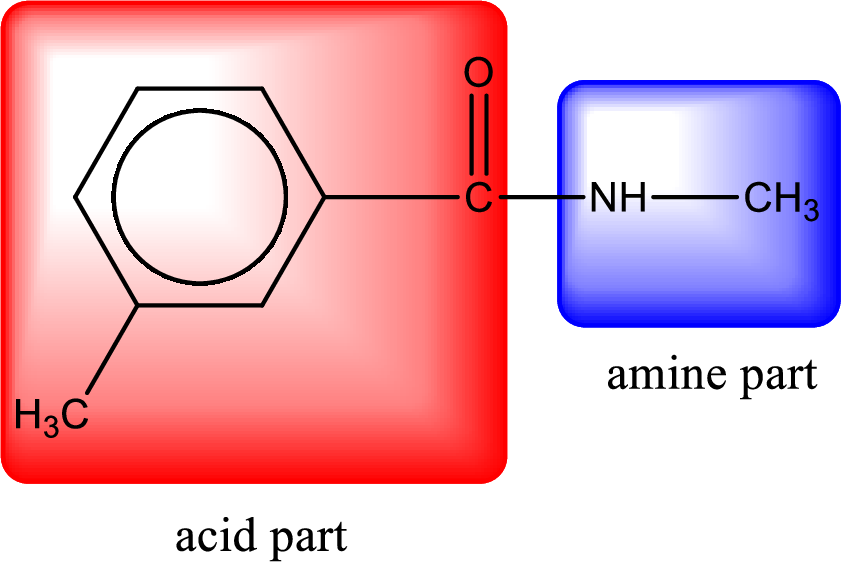
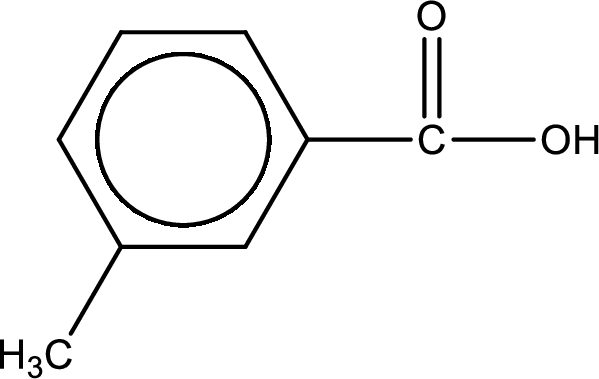
Hydrogen atom has to be added to the amine part and
The complete reaction can be given as,
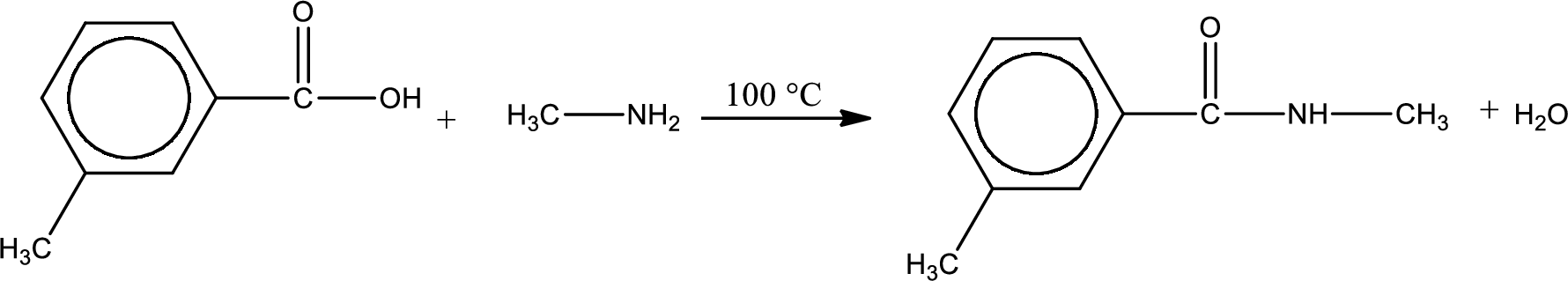
Structure of the missing compound is drawn.
Want to see more full solutions like this?
Chapter 6 Solutions
Organic And Biological Chemistry
- H CH3 CH3 b) Write the products of your compound and the following reagents. If the reaction would not work for your compound, write "no reaction" and explain the problem. NaCN H* H₂NNHCH5 H* -à NaBH -à CH2MgBr Cro₁₂ --à H3O+ -à c) Would your compound give a positive Tollen's test? Why or why not?arrow_forwardHomework 4 Chem 204 Dr. Hellwig Consider this compound, which will be referred to as "your compound". a) Name your compound according to the IUPAC system. Include stereochemistry (E/Z/R/S) H CH3 CH3arrow_forwardWhat is the mechanism for this?arrow_forward
- 21.50 Determine the combinations of haloalkane(s) and alkoxide(s) that could be used to synthesize the following ethers through Williamson ether synthesis. (a) (c) (d) (e) (f) H₂COarrow_forward1. Arrange the following in order of increasing bond energy (lowest bond energy first, highest bond energy last). Provide your rationale. C=C, C-F, C=C, C-N, C-C List the bond order for each example.arrow_forwardWhat is the major enolate formed when treated with LDA? And why that one?arrow_forward
- 4. Calculate the total number of sigma bonds and total number of pi bonds in each of the following compounds. a. HH :D: +1 I H-N-C-C-O-H I H b. HH H Н :N=C-C-C=C-CEC-H :0: total o H-C-H H-C = `C-H I H. 11 H-C = C= CH H total o total π total π 1 Harrow_forwardIn the following reaction, what quantity in moles of CH₃OH are required to give off 4111 kJ of heat? 2 CH₃OH (l) + 3 O₂ (g) → 2 CO₂ (g) + 4 H₂O(g) ∆H° = -1280. kJarrow_forwardIndicate the processes in the dismutation of Cu2O.arrow_forward
- 1. Consider these three reactions as the elementary steps in the mechanism for a chemical reaction. 2600 2400 2200 2000 1800 1600 1400 1200 1000 800 Potential Energy (kJ) 600 400 200 0 -200- -400 -600- -800 (i) Cl₂ (g) + Pt(s) → 2Cl (g) + Pt(s) (ii) Cl (g)+ CO (g) + Pt (s) → CICO (g) + Pt (s) Ea = 1550 kJ Ea = 2240 kJ (iii) Cl (g) + CICO (g) → Cl₂CO (g) Ea = 2350 kJ AH=-950 kJ ΔΗ = 575 ΚΙ AH=-825 kJ a. Draw the potential energy diagram for the reaction. Label the data points for clarity. The potential energy of the reactants is 600 kJ Reaction Progress b. What is the overall chemical equation? c. What is the overall change in enthalpy for the above chemical reaction? d. What is the overall amount of activation energy for the above chemical reaction? e. Which reaction intermediate would be considered a catalyst (if any) and why? f. If you were to add 2700kJ of energy to the reaction (e.g. 2700 kl of heat or electricity), would you be able to make the reaction reverse itself (i.e. have…arrow_forwarddraw the enolate anion and the carbonyl that would be needed to make this product through an aldol addition reaction.arrow_forwardDraw the Michael Adduct and the final product of the Robinson annulation reaction. Ignore inorganic byproducts.arrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning



