
(a)
Interpretation:
Structure of organic product that is obtained in the given hydrolysis reaction of given amide has to be drawn.
Concept Introduction:
Amides are derivatives of
General scheme of hydrolysis of an amide can be given as,
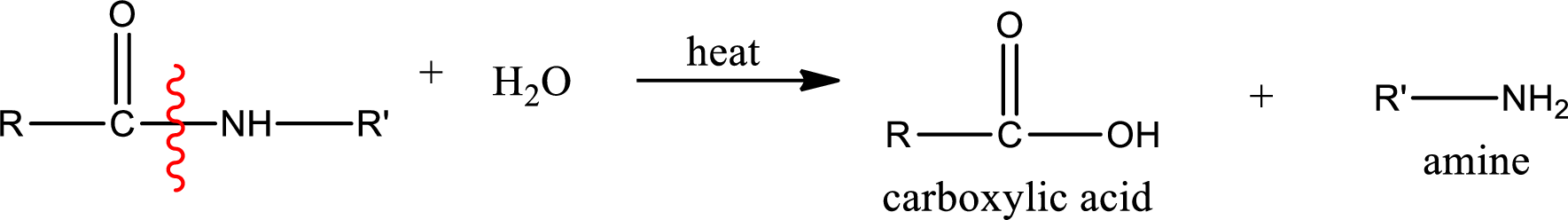
Acidic hydrolysis of amides gives the product as carboxylic acid and
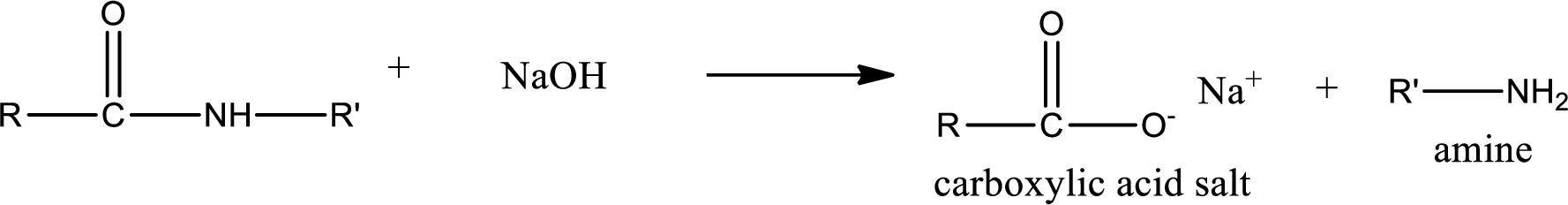
Basic hydrolysis of amides gives the product as carboxylic acid salt and amine. Carboxylic acid salt is obtained because in basic conditions the carboxylic acid is converted into carboxylic acid salt.
(b)
Interpretation:
The structure of organic products that are obtained when the given amide undergoes acidic hydrolysis has to be drawn.
Concept Introduction:
Amides are derivatives of carboxylic acid. Amides are not much reactive as of carboxylic acids. They are also stable relatively in aqueous solution. But under strenuous conditions amides undergo hydrolysis. The conditions are presence of acid, base or enzymes.
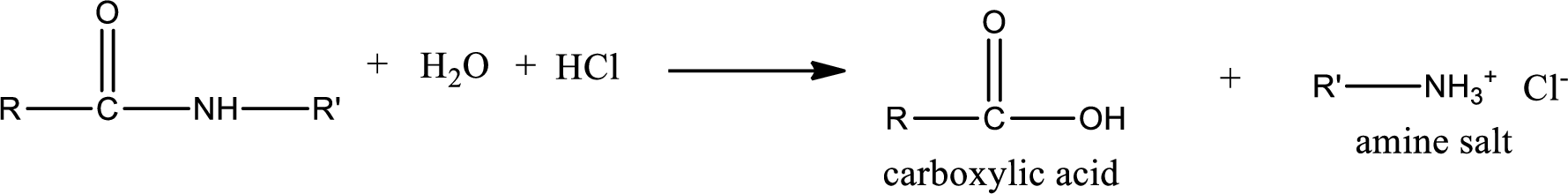
Acidic hydrolysis of amides gives the product as carboxylic acid and amine salt. Amine salt is obtained because in acidic conditions the amine is converted into amine salt.
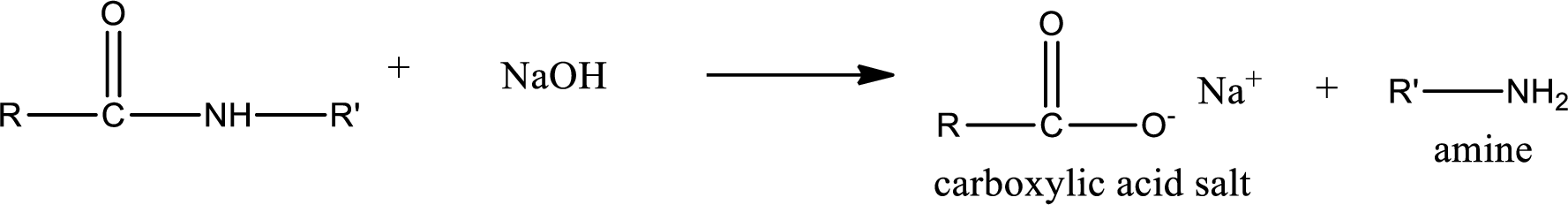
Basic hydrolysis of amides gives the product as carboxylic acid salt and amine. Carboxylic acid salt is obtained because in basic conditions the carboxylic acid is converted into carboxylic acid salt.
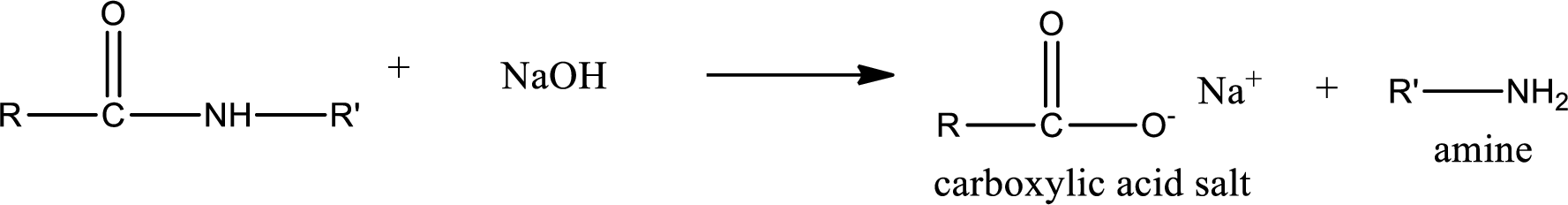
(c)
Interpretation:
The structure of organic products that are obtained when the given amide undergoes basic hydrolysis has to be drawn.
Concept Introduction:
Amides are derivatives of carboxylic acid. Amides are not much reactive as of carboxylic acids. They are also stable relatively in aqueous solution. But under strenuous conditions amides undergo hydrolysis. The conditions are presence of acid, base or enzymes.
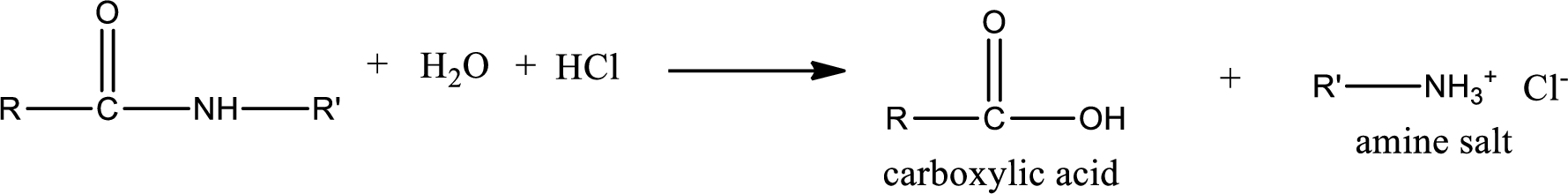
Acidic hydrolysis of amides gives the product as carboxylic acid and amine salt. Amine salt is obtained because in acidic conditions the amine is converted into amine salt.
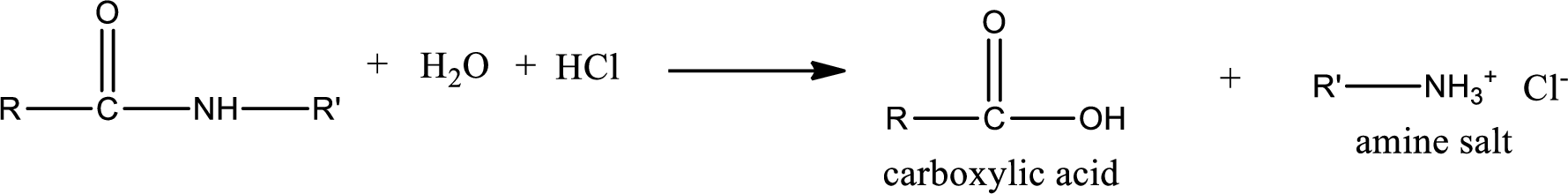
Basic hydrolysis of amides gives the product as carboxylic acid salt and amine. Carboxylic acid salt is obtained because in basic conditions the carboxylic acid is converted into carboxylic acid salt.
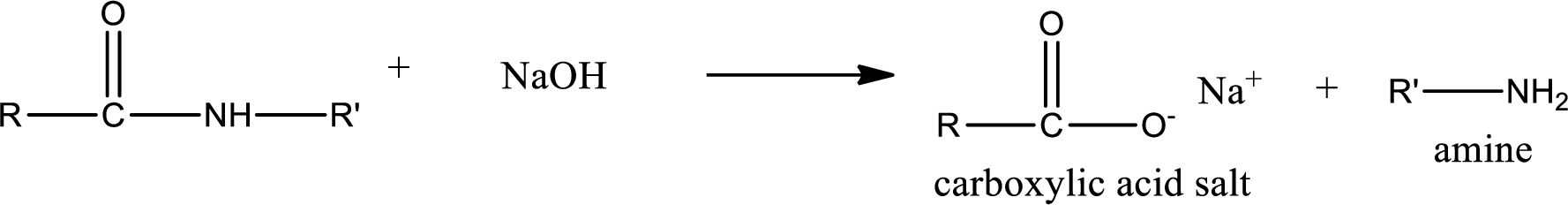
(d)
Interpretation:
Structure of organic product that is obtained in the given hydrolysis reaction of given amide has to be drawn.
Concept Introduction:
Amides are derivatives of carboxylic acid. Amides are not much reactive as of carboxylic acids. They are also stable relatively in aqueous solution. But under strenuous conditions amides undergo hydrolysis. The conditions are presence of acid, base or enzymes.
General scheme of hydrolysis of an amide can be given as,
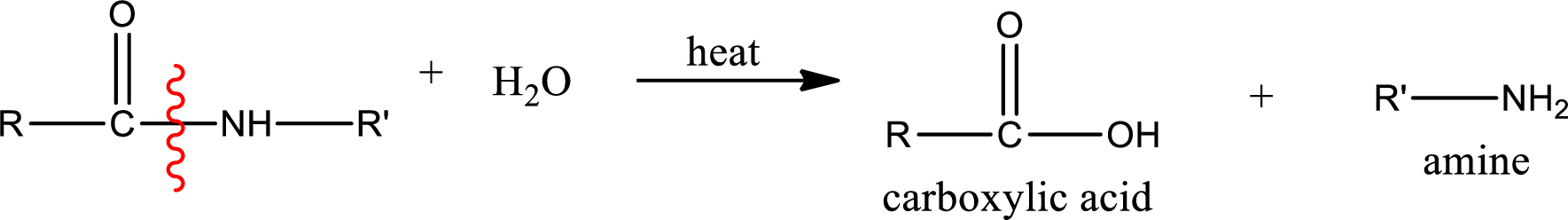
Acidic hydrolysis of amides gives the product as carboxylic acid and amine salt. Amine salt is obtained because in acidic conditions the amine is converted into amine salt.
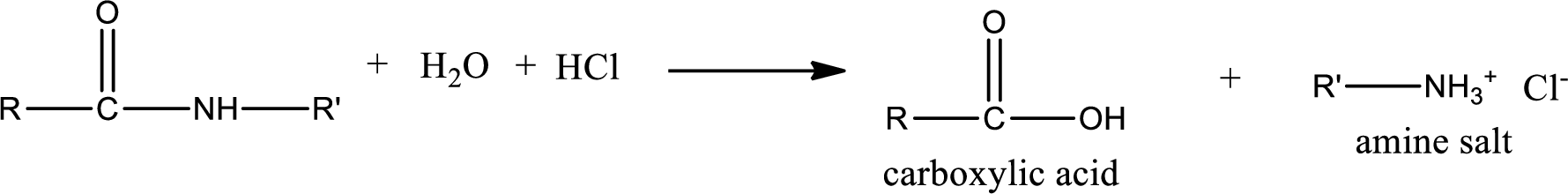
Basic hydrolysis of amides gives the product as carboxylic acid salt and amine. Carboxylic acid salt is obtained because in basic conditions the carboxylic acid is converted into carboxylic acid salt.
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
Organic And Biological Chemistry
- Using the Nernst equation to calculate nonstandard cell voltage A galvanic cell at a temperature of 25.0 °C is powered by the following redox reaction: 3+ 3Cu²+ (aq) +2Al(s) → 3 Cu(s)+2A1³* (aq) 2+ Suppose the cell is prepared with 5.29 M Cu in one half-cell and 2.49 M A1³+ in the other. Calculate the cell voltage under these conditions. Round your answer to 3 significant digits. x10 μ ☑ 00. 18 Ar Иarrow_forwardPlease help me solve this homework problemarrow_forwardPlease help me answer this homework questionarrow_forward
- Calculating standard reaction free energy from standard reduction... Using standard reduction potentials from the ALEKS Data tab, calculate the standard reaction free energy AG° for the following redox reaction. Be sure your answer has the correct number of significant digits. 3+ H2(g)+2OH¯ (aq) + 2Fe³+ (aq) → 2H₂O (1)+2Fe²+ (aq) 0 kJ x10 Х ? olo 18 Ararrow_forwardCalculating the pH of a weak base titrated with a strong acid An analytical chemist is titrating 184.2 mL of a 0.7800M solution of dimethylamine ((CH3) NH with a 0.3000M solution of HClO4. The pK₁ of dimethylamine is 3.27. Calculate the pH of the base solution after the chemist has added 424.1 mL of the HClO solution to it. 2 4 Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HClO 4 solution added. Round your answer to 2 decimal places. pH = ☐ ☑ ? 000 18 Ar 1 Barrow_forwardUsing the Nernst equation to calculate nonstandard cell voltage A galvanic cell at a temperature of 25.0 °C is powered by the following redox reaction: MnO2 (s)+4H* (aq)+2Cr²+ (aq) → Mn²+ (aq)+2H₂O (1)+2Cr³+ (aq) + 2+ 2+ 3+ Suppose the cell is prepared with 7.44 M H* and 0.485 M Cr²+ in one half-cell and 7.92 M Mn² and 3.73 M Cr³+ in the other. Calculate the cell voltage under these conditions. Round your answer to 3 significant digits. ☐ x10 μ Х 5 ? 000 日。arrow_forward
- Calculating standard reaction free energy from standard reduction... Using standard reduction potentials from the ALEKS Data tab, calculate the standard reaction free energy AG° for the following redox reaction. Be sure your answer has the correct number of significant digits. NO (g) +H₂O (1) + Cu²+ (aq) → HNO₂ (aq) +H* (aq)+Cu* (aq) kJ - ☐ x10 x10 olo 18 Ararrow_forwardCalculating the pH of a weak base titrated with a strong acid b An analytical chemist is titrating 116.9 mL of a 0.7700M solution of aniline (C6H5NH2) with a 0.5300M solution of HNO3. The pK of aniline is 9.37. Calculate the pH of the base solution after the chemist has added 184.2 mL of the HNO 3 solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HNO3 solution added. Round your answer to 2 decimal places. pH = ☐ ☑ 5arrow_forwardQUESTION: Find the standard deviation for the 4 different groups 5.298 3.977 223.4 148.7 5.38 4.24 353.7 278.2 5.033 4.044 334.6 268.7 4.706 3.621 305.6 234.4 4.816 3.728 340.0 262.7 4.828 4.496 304.3 283.2 4.993 3.865 244.7 143.6 STDEV = STDEV = STDEV = STDEV =arrow_forward
- QUESTION: Fill in the answers in the empty green boxes regarding 'Question 5: Calculating standard error of regression' *The images of the data showing 'coefficients for the standard curve' have been providedarrow_forwardUsing the Nernst equation to calculate nonstandard cell voltage Try Again Your answer is wrong. In addition to checking your math, check that you used the right data and DID NOT round any intermediate calculations. A galvanic cell at a temperature of 25.0 °C is powered by the following redox reaction: 2+ 2+ Sn²+ Ba(s) (aq) + Ba (s) Sn (s) + Ba²+ (aq) →>> Suppose the cell is prepared with 6.10 M Sn 2+ 2+ in one half-cell and 6.62 M Ba in the other. Calculate the cell voltage under these conditions. Round your answer to 3 significant digits. 1.71 V ☐ x10 ☑ 5 0/5 ? 00. 18 Ararrow_forwardQuestion: Find both the b (gradient) and a (y-intercept) value from the list of data below: (x1 -x̄) 370.5 (y1 - ȳ) 5.240 (x2 - x̄) 142.5 (y2 - ȳ) 2.004 (x3 - x̄) 28.5 (y3 - ȳ) 0.390 (x4 - x̄) -85.5 (y4 - ȳ) -1.231 (x5 - x̄) -199.5 (y5 - ȳ) -2.829 (x6 - x̄) -256.5 (y6 - ȳ) -3.575arrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning


