
(a)
Interpretation:
The structure of carbonyl-containing compound that is obtained when the given amide undergoes acidic hydrolysis has to be drawn.
Concept Introduction:
Amides are derivatives of
Acidic hydrolysis of amides gives the product as carboxylic acid and
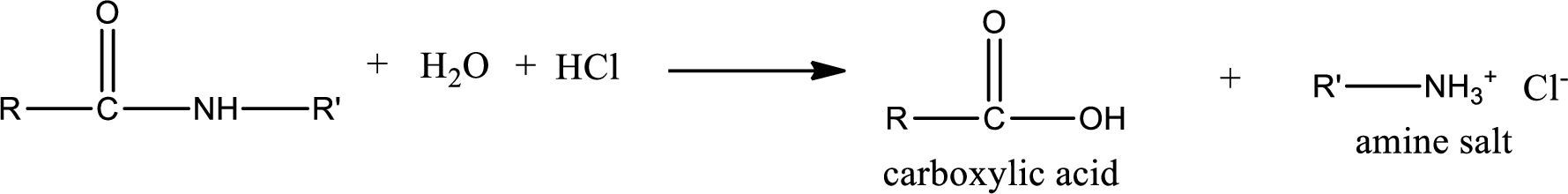
Basic hydrolysis of amides gives the product as carboxylic acid salt and amine. Carboxylic acid salt is obtained because in basic conditions the carboxylic acid is converted into carboxylic acid salt.
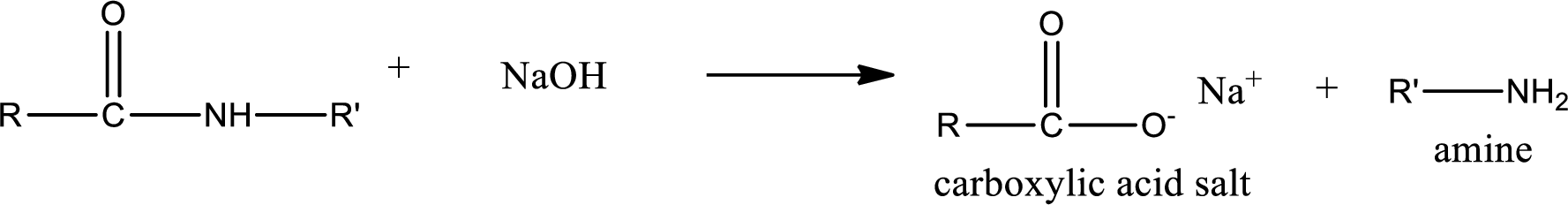
(b)
Interpretation:
The structure of carbonyl-containing compound that is obtained when the given amide undergoes acidic hydrolysis has to be drawn.
Concept Introduction:
Amides are derivatives of carboxylic acid. Amides are not much reactive as of carboxylic acids. They are also stable relatively in aqueous solution. But under strenuous conditions amides undergo hydrolysis. The conditions are presence of acid, base or enzymes.
Acidic hydrolysis of amides gives the product as carboxylic acid and amine salt. Amine salt is obtained because in acidic conditions the amine is converted into amine salt.
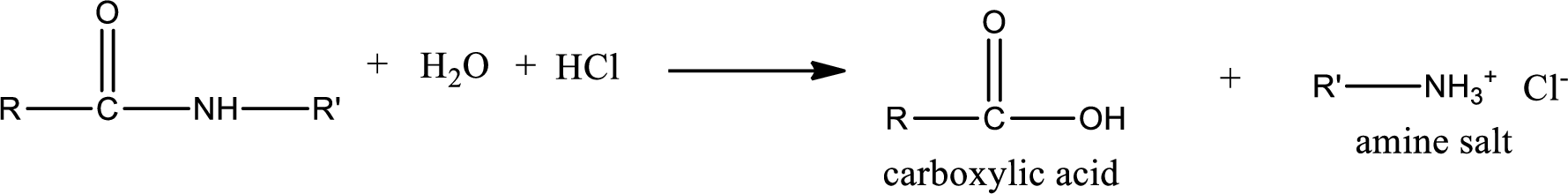
Basic hydrolysis of amides gives the product as carboxylic acid salt and amine. Carboxylic acid salt is obtained because in basic conditions the carboxylic acid is converted into carboxylic acid salt.
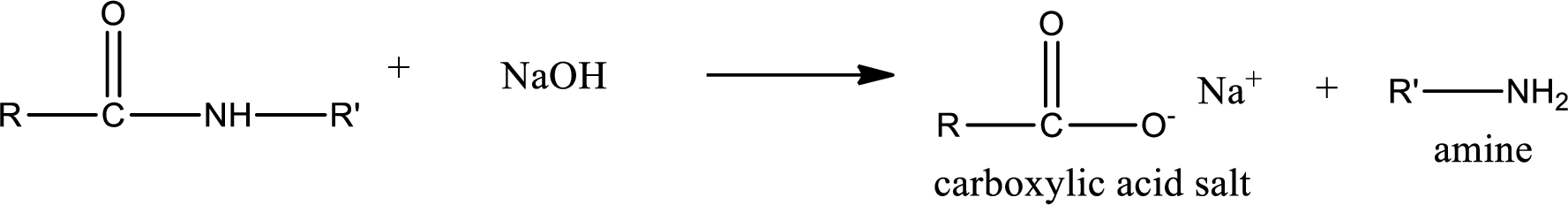
(c)
Interpretation:
The structure of carbonyl-containing compound that is obtained when the given amide undergoes acidic hydrolysis has to be drawn.
Concept Introduction:
Amides are derivatives of carboxylic acid. Amides are not much reactive as of carboxylic acids. They are also stable relatively in aqueous solution. But under strenuous conditions amides undergo hydrolysis. The conditions are presence of acid, base or enzymes.
Acidic hydrolysis of amides gives the product as carboxylic acid and amine salt. Amine salt is obtained because in acidic conditions the amine is converted into amine salt.
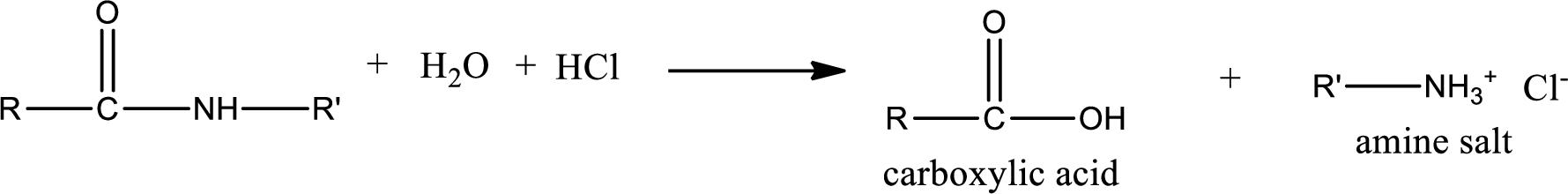
Basic hydrolysis of amides gives the product as carboxylic acid salt and amine. Carboxylic acid salt is obtained because in basic conditions the carboxylic acid is converted into carboxylic acid salt.
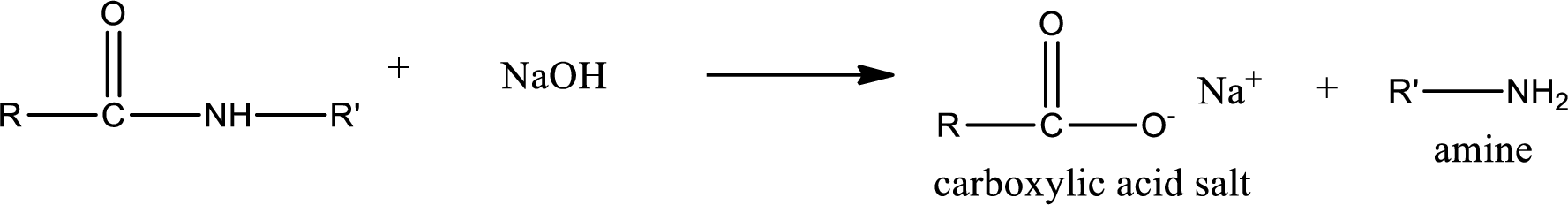
(d)
Interpretation:
The structure of carbonyl-containing compound that is obtained when the given amide undergoes acidic hydrolysis has to be drawn.
Concept Introduction:
Amides are derivatives of carboxylic acid. Amides are not much reactive as of carboxylic acids. They are also stable relatively in aqueous solution. But under strenuous conditions amides undergo hydrolysis. The conditions are presence of acid, base or enzymes.
Acidic hydrolysis of amides gives the product as carboxylic acid and amine salt. Amine salt is obtained because in acidic conditions the amine is converted into amine salt.
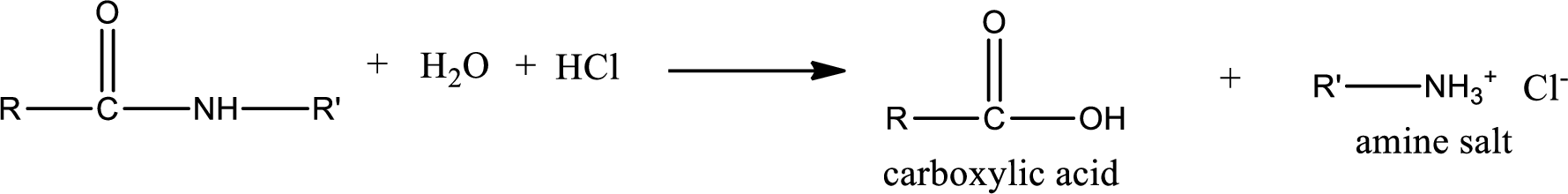
Basic hydrolysis of amides gives the product as carboxylic acid salt and amine. Carboxylic acid salt is obtained because in basic conditions the carboxylic acid is converted into carboxylic acid salt.
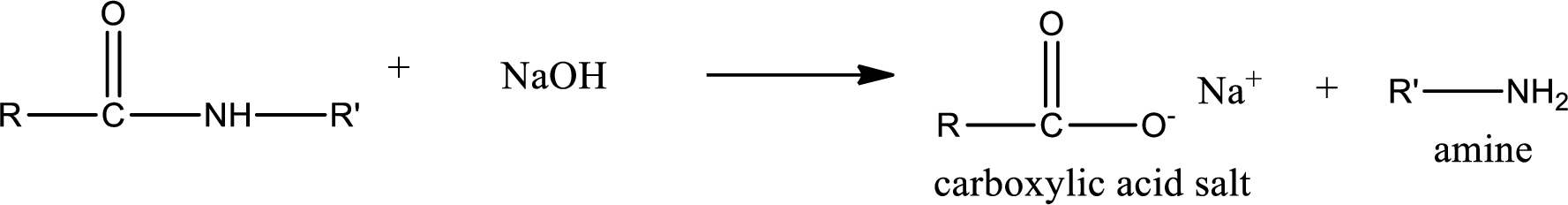
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
Organic And Biological Chemistry
- The temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forward
- er your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward5.arrow_forward6.arrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning


