
Concept explainers
(a)
Interpretation:
The “
Concept Introduction:
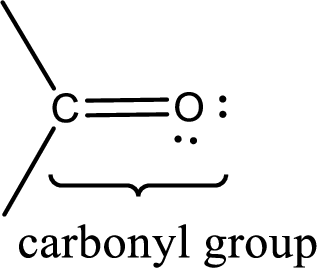
Carbonyl groups are the one which contain a double bond between carbon and oxygen atom.
If a hydroxyl group is attached to a carbonyl group means it is known as carboxyl group. This can be represented as shown below,
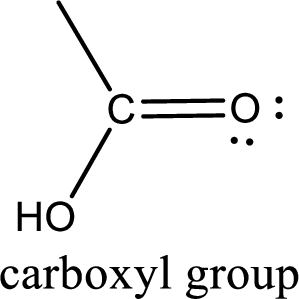
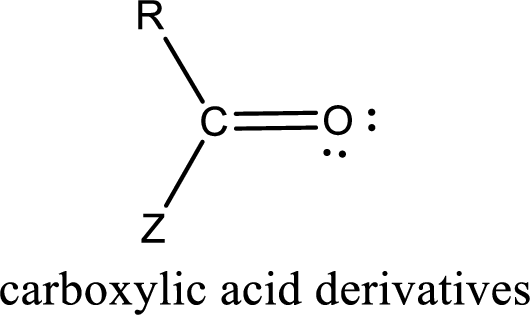
In the carboxylic acid derivatives, if the carbonyl carbon atom is bonded to a more electronegative atom means, then the bond will be polar and is it is bonded to carbon atom means then it will be nonpolar.
(a)
Answer to Problem 5.8EP
The “
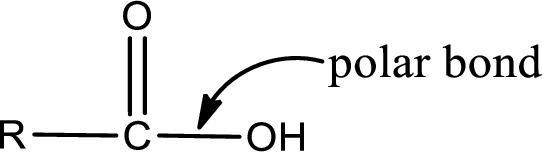
Explanation of Solution
The general structure of carboxylic acid is,
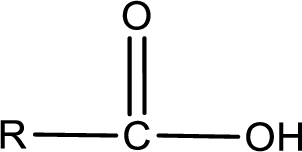
The atom in entity Z that is bonded to the carbonyl carbon atom is not a carbon atom. It is an oxygen atom. As there is a polarity difference between carbon and oxygen atom, the bond between carbon and oxygen will be polar. This can be shown as given below,
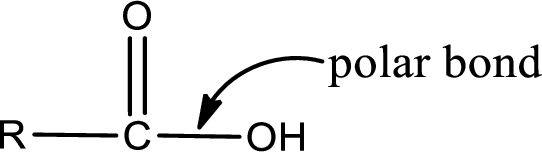
The “
(b)
Interpretation:
The “
Concept Introduction:
Carbonyl groups are the one which contain a double bond between carbon and oxygen atom. Aldehydes and ketones possess this carbonyl functional group in it. The structural representation of a carbonyl group can be given as shown below,
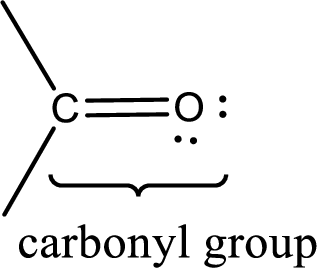
If a hydroxyl group is attached to a carbonyl group means it is known as carboxyl group. This can be represented as shown below,
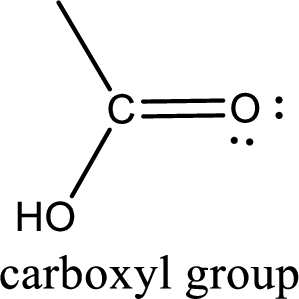
Carboxylic acid derivatives are the ones that are synthesized from or converted to a carboxylic acid. The generalized structural representation of carboxylic acid derivatives is shown below,
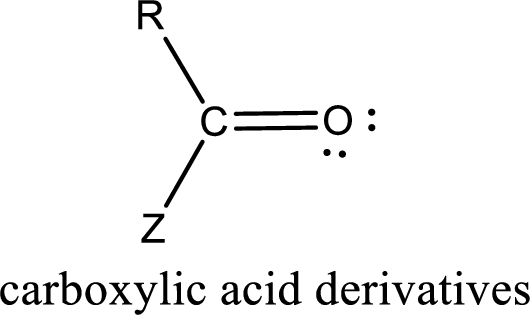
In the carboxylic acid derivatives, if the carbonyl carbon atom is bonded to a more electronegative atom means, then the bond will be polar and is it is bonded to carbon atom means then it will be nonpolar.
(b)
Answer to Problem 5.8EP
The “
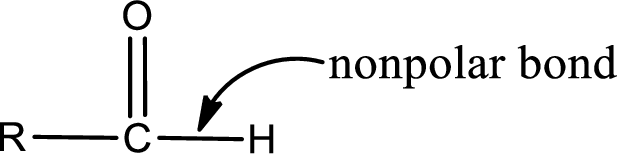
Explanation of Solution
The general structure of carboxylic acid derivatives is,
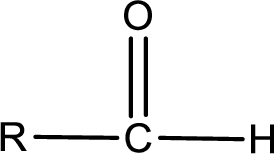
The atom in entity Z that is bonded to the carbonyl carbon atom is a hydrogen atom. Hydrogen atom is not a heteroatom and hence the bond between carbonyl carbon atom and hydrogen atom is said to be nonpolar. This can be shown as given below,
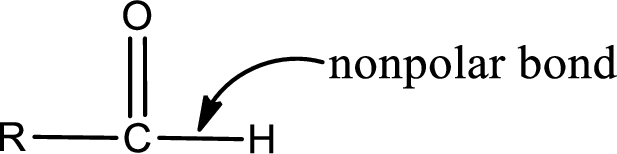
The “
(c)
Interpretation:
The “
Concept Introduction:
Carbonyl groups are the one which contain a double bond between carbon and oxygen atom. Aldehydes and ketones possess this carbonyl functional group in it. The structural representation of a carbonyl group can be given as shown below,
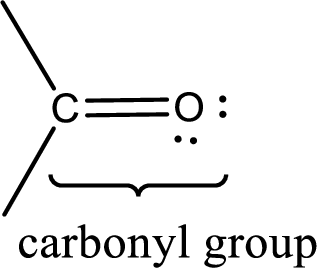
If a hydroxyl group is attached to a carbonyl group means it is known as carboxyl group. This can be represented as shown below,
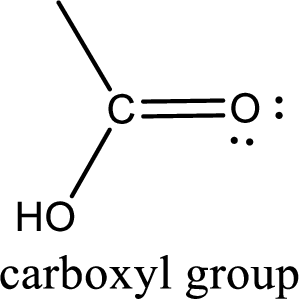
Carboxylic acid derivatives are the ones that are synthesized from or converted to a carboxylic acid. The generalized structural representation of carboxylic acid derivatives is shown below,
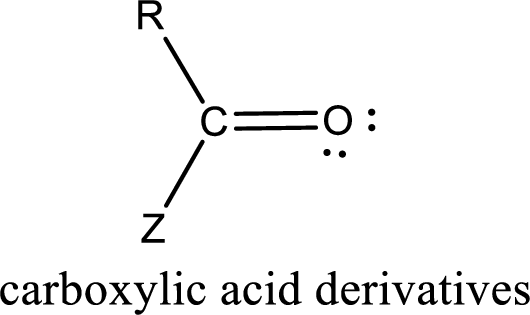
In the carboxylic acid derivatives, if the carbonyl carbon atom is bonded to a more electronegative atom means, then the bond will be polar and is it is bonded to carbon atom means then it will be nonpolar.
(c)
Answer to Problem 5.8EP
The “
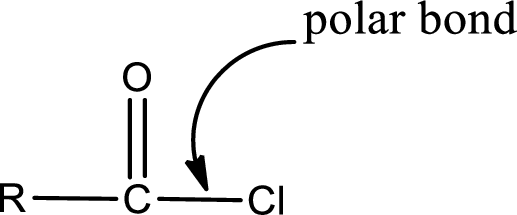
Explanation of Solution
The general structure of carboxylic acid derivatives is,
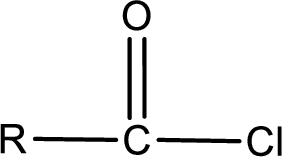
The atom in entity Z that is bonded to the carbonyl carbon atom is a chlorine atom. As there is a polarity difference between carbon and chlorien atom, the bond between carbon and chlorine will be polar. This can be shown as given below,
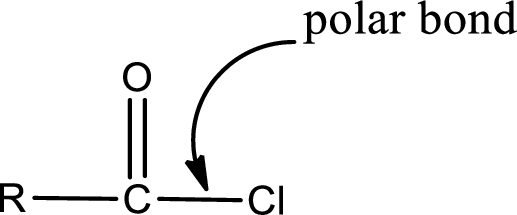
The “
(d)
Interpretation:
The “
Concept Introduction:
Carbonyl groups are the one which contain a double bond between carbon and oxygen atom. Aldehydes and ketones possess this carbonyl functional group in it. The structural representation of a carbonyl group can be given as shown below,
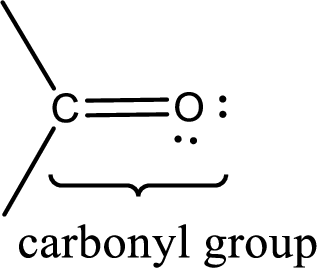
If a hydroxyl group is attached to a carbonyl group means it is known as carboxyl group. This can be represented as shown below,
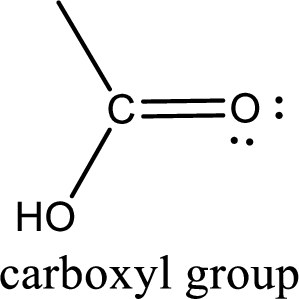
Carboxylic acid derivatives are the ones that are synthesized from or converted to a carboxylic acid. The generalized structural representation of carboxylic acid derivatives is shown below,
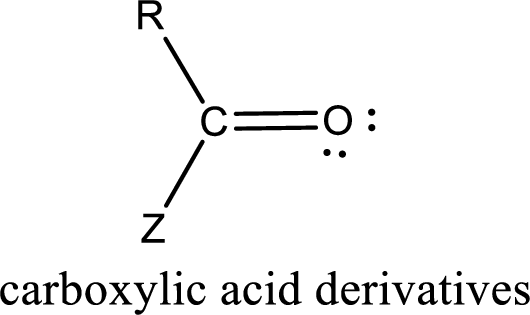
In the carboxylic acid derivatives, if the carbonyl carbon atom is bonded to a more electronegative atom means, then the bond will be polar and is it is bonded to carbon atom means then it will be nonpolar.
(d)
Answer to Problem 5.8EP
The “
Explanation of Solution
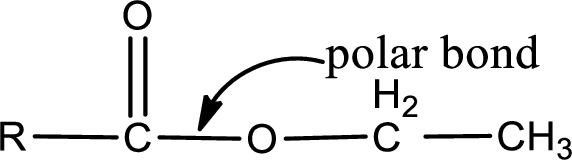
The general structure of carboxylic acid derivatives is,
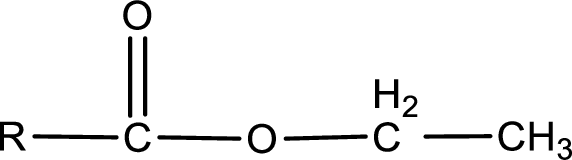
The atom in entity Z that is bonded to the carbonyl carbon atom is an oxygen atom. As there is a polarity difference between carbon and oxygen atom, the bond between carbon and oxygen will be polar. This can be shown as given below,
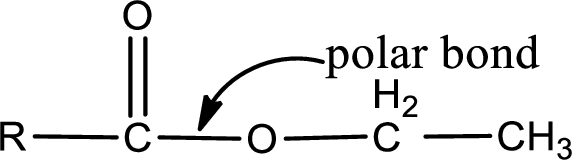
The “
Want to see more full solutions like this?
Chapter 5 Solutions
EBK ORGANIC AND BIOLOGICAL CHEMISTRY
- Can I get helpp drawing my arrowsarrow_forwardWhich of the m/z values corresponds to the base peak in the mass spectrum shown? 100 80 A. 45 B. 44 C. 29 D. 15 Intensity 20 0 10 20 30 40 B- m/z -8 50 E. 30 Which of the m/z values correspond to the molecular ion for the compound shown? A. 18 B. 82 OH C. 100 D. 102 E. 103arrow_forwardCan someone help me with drawing my arrows.arrow_forward
- I'm having trouble with converting lewis diagrams into VSEPR diagrams. I currently have this example of C2BrCl3 which I want to turn into a lewis structure, but I'm not sure what steps I need to do in order to do so. I have the table written down, however, there's two central atoms so what would I do? There seems to be 4 electron domains on the carbon atom and no lone pairs so it would seem like this shape would be tetrahedral. Here's what I have now. Thanks!arrow_forwardWe discussed the solid phase resin using in peptide synthesis. Provide a mechanism, for its formation. DRAW THE MECHANISM.arrow_forwardPlease help. Every time I've asked an expert in the past, it's been wrong :(arrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning





