
(a)
Interpretation:
Condensed structural formula for acetyl chloride has to be drawn.
Concept Introduction:
General structure of acid chloride can be represented as shown below,
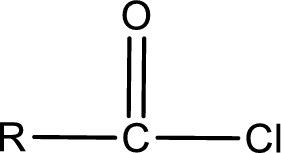
The structure of the acid chloride can be derived from the IUPAC name or common name. “R” group present in the above structure is a part of the parent
(b)
Interpretation:
Condensed structural formula for 2-methylbutanoyl chloride has to be drawn.
Concept Introduction:
General structure of acid chloride can be represented as shown below,
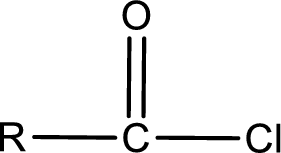
The structure of the acid chloride can be derived from the IUPAC name or common name. “R” group present in the above structure is a part of the parent carboxylic acid from which the acid chloride is obtained. Name of the acid chloride is obtained by replacing “-oic acid” in the parent carboxylic acid name with “-oyl chloride”.
(c)
Interpretation:
Condensed structural formula for propionic anhydride has to be drawn.
Concept Introduction:
General structure of acid anhydride can be represented as shown below,
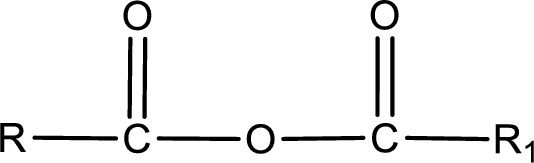
The structure of the acid anhydride can be derived from the IUPAC name or common name. “R, R1” group present in the above structure is a part of the parent carboxylic acid from which the acid anhydride is obtained. If the acid anhydride is a symmetric one, then the name of acid anhydride is given by replacing the acid in the parent carboxylic acid name with anhydride. If the acid anhydride is an asymmetric one, then the name of acid anhydride is given by using the name of individual carboxylic acid in an alphabetical order followed by anhydride.
(d)
Interpretation:
Condensed structural formula for ethanoic methanoic anhydride has to be drawn.
Concept Introduction:
General structure of acid anhydride can be represented as shown below,
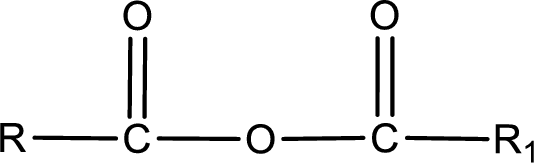
The structure of the acid anhydride can be derived from the IUPAC name or common name. “R, R1” group present in the above structure is a part of the parent carboxylic acid from which the acid anhydride is obtained. If the acid anhydride is a symmetric one, then the name of acid anhydride is given by replacing the acid in the parent carboxylic acid name with anhydride. If the acid anhydride is an mixed one, then the name of acid anhydride is given by using the name of individual carboxylic acid in an alphabetical order followed by anhydride.
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
EBK ORGANIC AND BIOLOGICAL CHEMISTRY
- The reaction Q(g) + R(g) → Z(l) is shown to be exothermic. Which of the following is true concerning the reactionarrow_forwardWhich of the following has the largest standard molar entropy, S° (298.15 K) He H2 NaCl KBr Hgarrow_forwardWhich of the following is true for a particular reaction if ∆G° is -40.0 kJ/mol at 290 K and –20.0 kJ/mol at 390 K?arrow_forward
- Choose the major product of the reaction with correct regio- and stereochemistry. Br2 H₂O O "Br Br & O 'Br OH Br 吡 O OH OH Br "OH Brarrow_forwardSelect the major product of the following reaction. & Br (CH)CONa (CH₂),COH 0 OC(CH) O &arrow_forwardDraw the products of the hydrolysis reaction between the ester molecule and water. Determine the products of the following reaction.arrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,





