
Concept explainers
(a)
Interpretation:
IUPAC name for the fumaric acid has to be given.
Concept Introduction:
For naming a
IUPAC rules for naming a carboxylic acid:
- The longest parent carbon chain is identified that includes the carboxyl group.
- The parent chain name is changed by replacing the suffix “-e” with “-oic acid”.
- Numbering is done in a way that the carboxyl group is designated as number 1. This is not indicated in the part of the name because for
aldehyde , the carboxyl carbon is always numbered 1. - The identity and location of substituents if any has to be determined and this information has to be added in front of the IUPAC name.
- If the carboxyl
functional group is attached to a ring of carbon atoms, the ring is named and “-carboxylic acid” is added as suffix. - If the compound contains two carboxyl groups, then suffix “-dioic acid” is added after the parent alkane name.
(a)
Answer to Problem 5.34EP
IUPAC name of fumaric acid is trans-butenedioic acid.
Explanation of Solution
Structure of fumaric acid is,
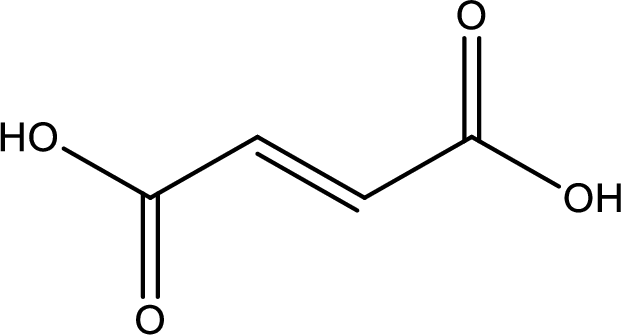
The longest continuous carbon chain has to be found out with the carboxyl group in it. In this it is a four carbon chain. The structure contains a double bond in it. The parent carbon chain is butene. The given structure contains two carboxyl groups. The carboxylic acid is named by adding suffix “-dioic acid”. This gives the name of carboxylic acid as butenedioic acid.
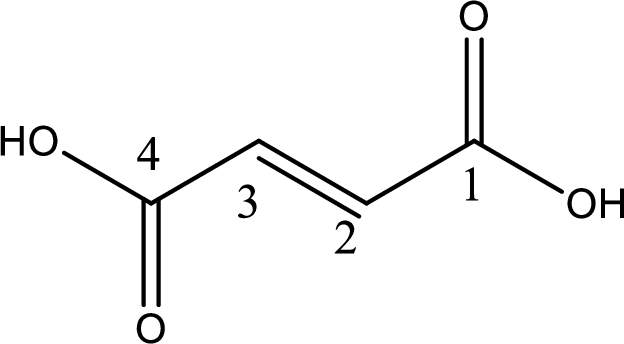
Looking for substituents it is found that there are no substituents present in the carbon chain. Stereochemistry is possible across the double bond. As the two hydrogen atoms are on the opposite side of double bond, the configuration at the double bond is “trans”. This has to be included in the name to get the IUPAC name. IUPAC name of the fumaric acid is found as trans-butenedioic acid.
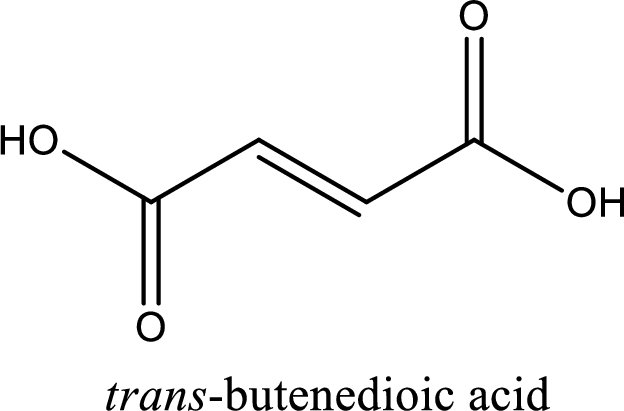
IUPAC name of fumaric acid is given.
(a)
Interpretation:
IUPAC name for the pyruvic acid has to be given.
Concept Introduction:
For naming a carboxylic acid in IUPAC nomenclature, the suffix “-oic” is added to the parent alkane name.
IUPAC rules for naming a carboxylic acid:
- The longest parent carbon chain is identified that includes the carboxyl group.
- The parent chain name is changed by replacing the suffix “-e” with “-oic acid”.
- Numbering is done in a way that the carboxyl group is designated as number 1. This is not indicated in the part of the name because for aldehyde, the carboxyl carbon is always numbered 1.
- The identity and location of substituents if any has to be determined and this information has to be added in front of the IUPAC name.
- If the carboxyl functional group is attached to a ring of carbon atoms, the ring is named and “-carboxylic acid” is added as suffix.
- If the compound contains two carboxyl groups, then suffix “-dioic acid” is added after the parent alkane name.
(a)
Answer to Problem 5.34EP
IUPAC name of pyruvic acid is 2-oxopropanoic acid.
Explanation of Solution
Structure of pyruvic acid is,
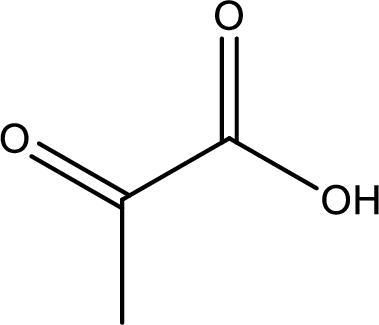
The longest continuous carbon chain has to be found out with the carboxyl group in it. In this it is a three carbon chain. The parent alkane is propane. The given structure contains a carboxyl group. The carboxylic acid is named by replacing the suffix “-e” with suffix “-oic acid”. This gives the name of carboxylic acid as propanoic acid.
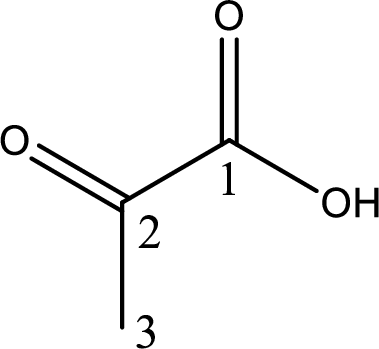
Looking for substituents it is found that there is a keto group present on the second carbon atom. Hence, the IUPAC name of the pyruvic acid is 2-oxopropanoic acid.
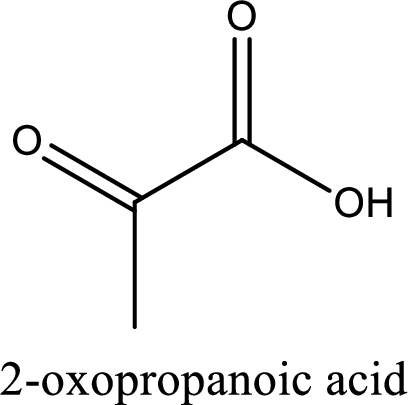
IUPAC name of pyruvic acid is given.
(c)
Interpretation:
IUPAC name for the malic acid has to be given.
Concept Introduction:
For naming a carboxylic acid in IUPAC nomenclature, the suffix “-oic” is added to the parent alkane name.
IUPAC rules for naming a carboxylic acid:
- The longest parent carbon chain is identified that includes the carboxyl group.
- The parent chain name is changed by replacing the suffix “-e” with “-oic acid”.
- Numbering is done in a way that the carboxyl group is designated as number 1. This is not indicated in the part of the name because for aldehyde, the carboxyl carbon is always numbered 1.
- The identity and location of substituents if any has to be determined and this information has to be added in front of the IUPAC name.
- If the carboxyl functional group is attached to a ring of carbon atoms, the ring is named and “-carboxylic acid” is added as suffix.
- If the compound contains two carboxyl groups, then suffix “-dioic acid” is added after the parent alkane name.
(c)
Answer to Problem 5.34EP
IUPAC name of malic acid is 2-hydroxybutanedioic acid.
Explanation of Solution
Structure of malic acid is,
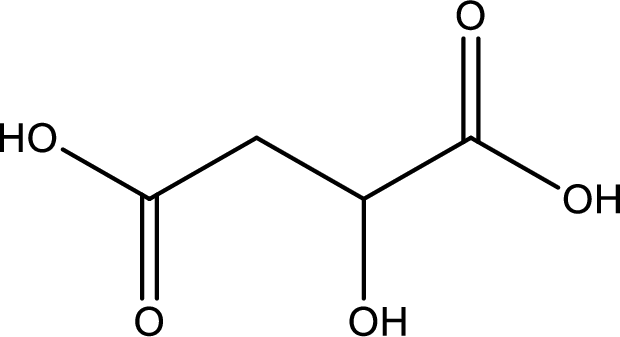
The longest continuous carbon chain has to be found out with the carboxyl group in it. In this it is a four carbon chain. The parent alkane is butane. The given structure contains two carboxyl groups. The carboxylic acid is named by adding the suffix “-dioic acid”. This gives the name of carboxylic acid as butanedioic acid.
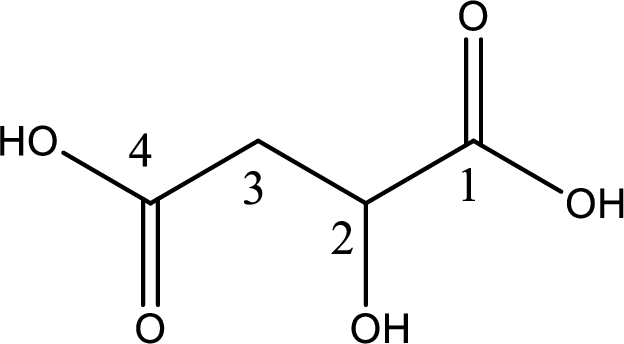
Looking for substituents it is found that there is a hydroxyl group at the second carbon atom. Hence, the IUPAC name of the malic acid is 2-hydroxybutanoic acid.
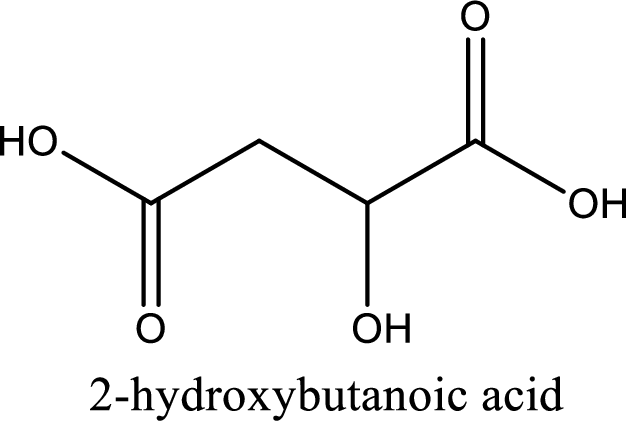
IUPAC name of malic acid is given.
(d)
Interpretation:
IUPAC name for the tartaric acid has to be given.
Concept Introduction:
For naming a carboxylic acid in IUPAC nomenclature, the suffix “-oic” is added to the parent alkane name.
IUPAC rules for naming a carboxylic acid:
- The longest parent carbon chain is identified that includes the carboxyl group.
- The parent chain name is changed by replacing the suffix “-e” with “-oic acid”.
- Numbering is done in a way that the carboxyl group is designated as number 1. This is not indicated in the part of the name because for aldehyde, the carboxyl carbon is always numbered 1.
- The identity and location of substituents if any has to be determined and this information has to be added in front of the IUPAC name.
- If the carboxyl functional group is attached to a ring of carbon atoms, the ring is named and “-carboxylic acid” is added as suffix.
- If the compound contains two carboxyl groups, then suffix “-dioic acid” is added after the parent alkane name.
(d)
Answer to Problem 5.34EP
IUPAC name of tartaric acid is 2,3-dihydroxybutanedioic acid.
Explanation of Solution
Structure of tartaric acid is,
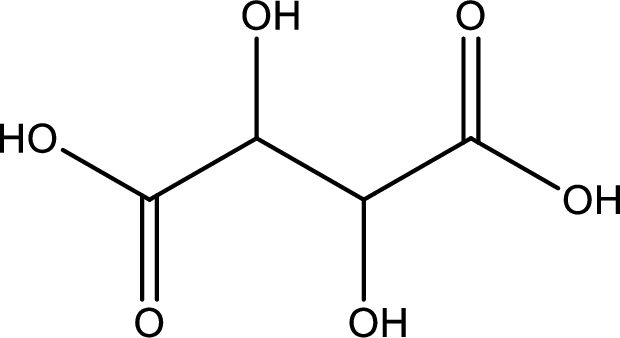
The longest continuous carbon chain has to be found out with the carboxyl group in it. In this it is a four carbon chain. The parent alkane is butane. The given structure contains two carboxyl groups. The carboxylic acid is named by adding the suffix “-dioic acid”. This gives the name of carboxylic acid as butanedioic acid.
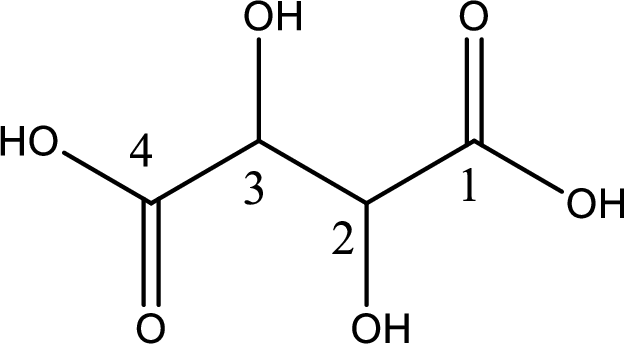
Looking for substituents it is found that there are two hydroxyl groups present, each at the second carbon atom and third carbon atom. Hence, the IUPAC name of the tartaric acid is 2,3-dihydroxybutanoic acid.
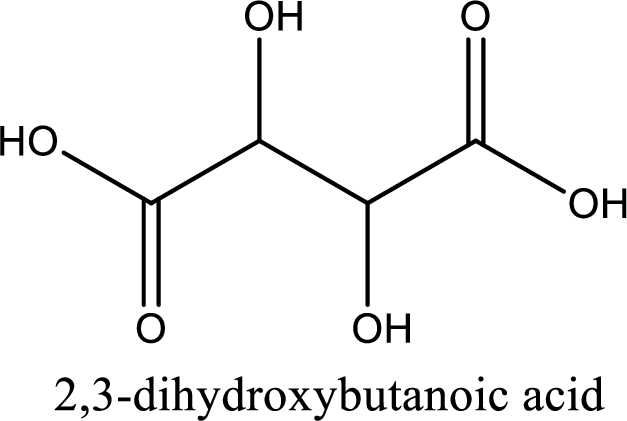
IUPAC name of tartaric acid is given.
Want to see more full solutions like this?
Chapter 5 Solutions
EBK ORGANIC AND BIOLOGICAL CHEMISTRY
- What will the enolate for this be using LDA, THF, and cold temperatures? What will it be using NaOEt at rt?arrow_forwardHelp me solve this problem.arrow_forwardDraw a mechanism for the following synthetic transformation including reagents and any isolable intermediates throughout the process. Please clearly indicate bond cleavage/formation using curly arrows. MeO2Carrow_forward
- CHEM 310 Quiz 8 Organic Chemistry II Due: Tuesday, April 25th, at 11:59 pm. This quiz is open textbook / open notes - but you must work alone. You cannot use the internet or the solutions manual for the book. Scan in your work and record an explanation of your mechanism. You may record this any way that you like. One way would be to start an individual Zoom meeting, start recording, "share your screen" and then talk through the problem. This will be converted to an .mp4 file that you can upload into Canvas using the "record/upload media" feature. Pyridine, benzoic acid and benzene are dissolved in ethyl acetate. Design and provide a plan / flow chart for separating and isolating each of these components. Pyridine and benzene are liquids at room temperature. Benzoic acid is a solid. You have ethyl acetate, 2M NaOH, 2M HCI and anhydrous MgSO4 available, as well as all the glassware and equipment that you used in the organic lab this year. Provide accurate acid/base reactions for any…arrow_forwardCan anyone help me solve this step by step. Thank you in advaarrow_forwardPlease draw the mechanism for this Friedel-crafts acylation reaction using arrowsarrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning





