
Concept explainers
(a)
Interpretation:
The IUPAC name for the ester formed when ethanoic acid and propyl alcohol react has to be assigned.
Concept Introduction:
Esters are prepared by condensation of
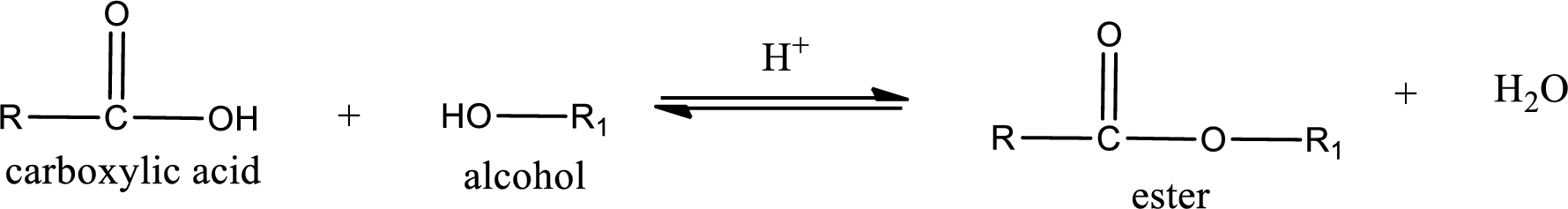
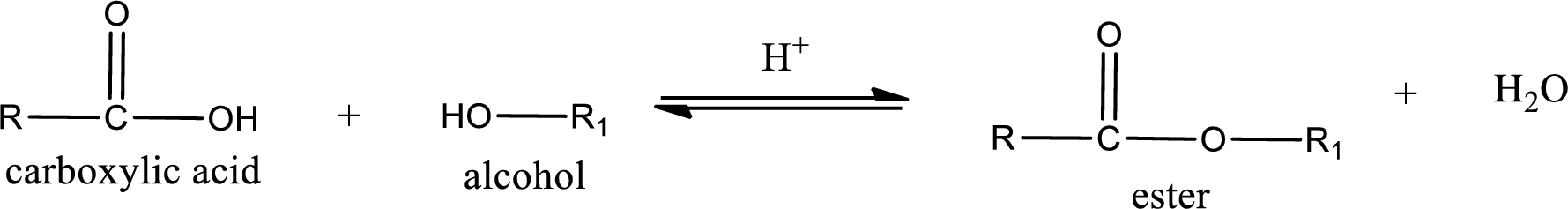
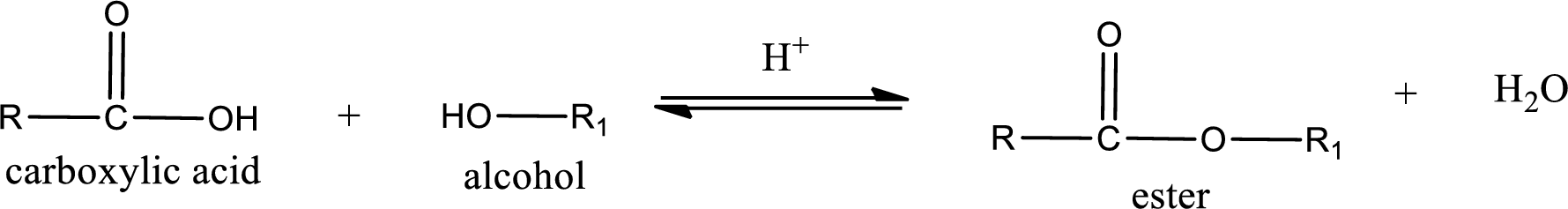
Esterification reaction is the one in which the carboxylic acid is condensed with an alcohol (or phenol) in presence of strong acid catalyst to produce ester. The general reaction scheme can be given as,
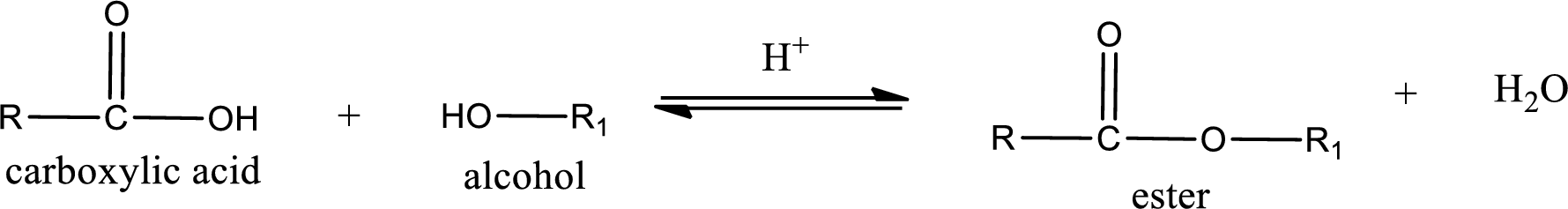
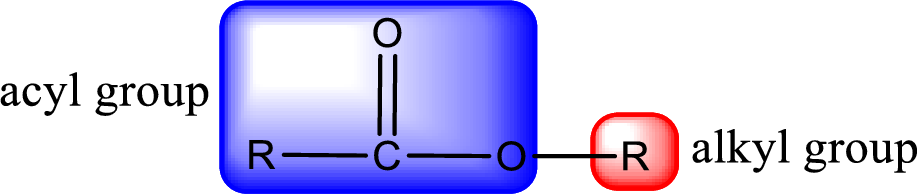
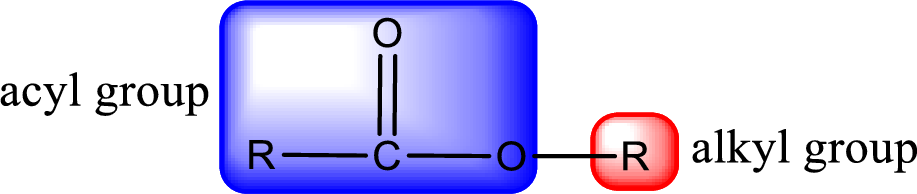
For naming an ester, it can be structurally viewed in a way that contains an acyl group and an alkyl group.
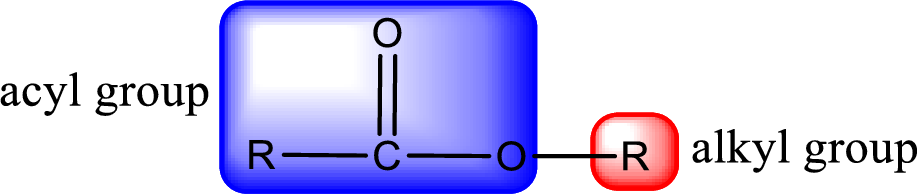
Rules to obtain IUPAC name and common name for an ester:
- Alkyl part appears first in the IUPAC name and it is followed by the acyl part of ester as a separate word.
- Name of the alkyl part in the ester is just a name of R group. It can be alkyl, cycloalkyl, or aryl group.
- Acyl part present in the ester is named by considering the acid name and replacing the suffix “-ic acid” with “-ate”.
- To obtain the common name the alkyl part name is the same while the acyl part name is derived from the common name of the acid by replacing the suffix “-ic acid” with “-ate”.
(a)
Answer to Problem 5.98EP
IUPAC name of the ester formed is propyl ethanoate.
Explanation of Solution
Given carboxylic acid and alcohol structure is,

The reaction between two compounds that are shown above, result in the formation of ester. The structure of the ester formed and the complete reaction can be given as shown below,
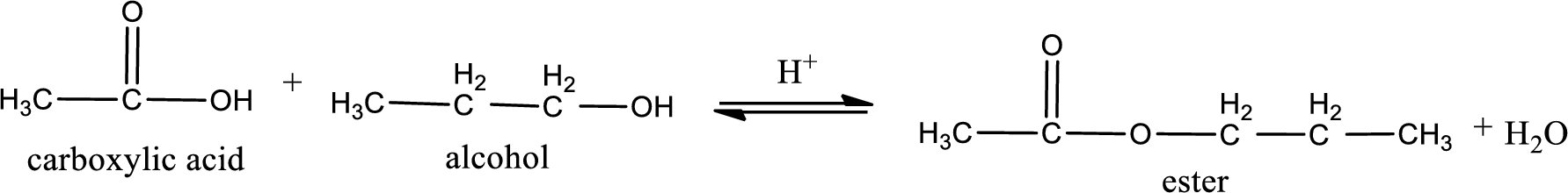
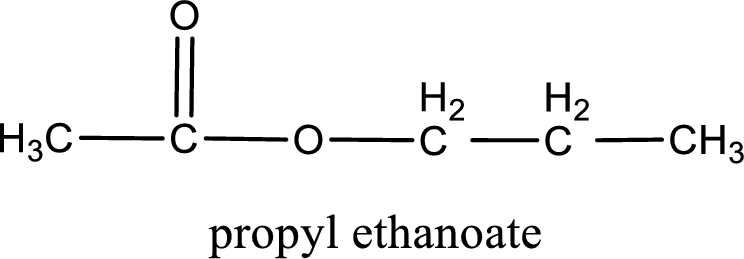
The structure of ester is,
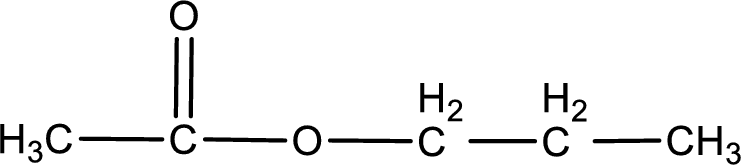
The alkyl and acyl group is identified as shown below,
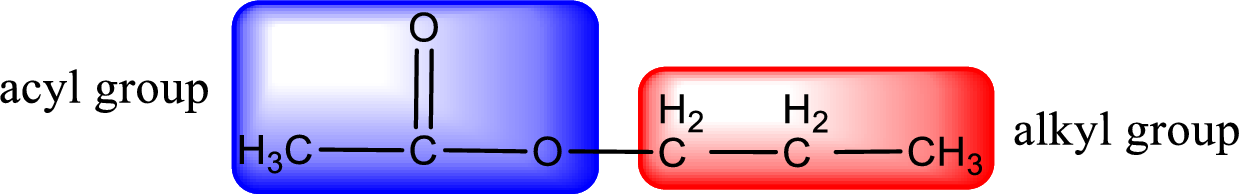
Alkyl group contains three carbon atoms. Hence alkyl group is named as propyl. The acyl group contains two carbon atoms. The IUPAC name of carboxylic acid that contains three carbon atoms is ethanoic acid. For naming acyl group in ester the suffix “-ic acid” is converted into “-ate”. This gives the name of acyl part as ethanoate. Therefore IUPAC name of the given ester is propyl ethanoate.
IUPAC name of the formed ester is assigned.
(b)
Interpretation:
The IUPAC name for the ester formed when acetic acid and 1-pentanol react has to be assigned.
Concept Introduction:
Esters are prepared by condensation of carboxylic acid with an alcohol. A molecule of water is lost on this reaction. The reaction that takes place in producing esters is known as esterification reaction.
Esterification reaction is the one in which the carboxylic acid is condensed with an alcohol (or phenol) in presence of strong acid catalyst to produce ester. The general reaction scheme can be given as,
For naming an ester, it can be structurally viewed in a way that contains an acyl group and an alkyl group.
Rules to obtain IUPAC name and common name for an ester:
- Alkyl part appears first in the IUPAC name and it is followed by the acyl part of ester as a separate word.
- Name of the alkyl part in the ester is just a name of R group. It can be alkyl, cycloalkyl, or aryl group.
- Acyl part present in the ester is named by considering the acid name and replacing the suffix “-ic acid” with “-ate”.
- To obtain the common name the alkyl part name is the same while the acyl part name is derived from the common name of the acid by replacing the suffix “-ic acid” with “-ate”.
(b)
Answer to Problem 5.98EP
IUPAC name of the ester formed is pentyl ethanoate.
Explanation of Solution
Given carboxylic acid and alcohol structure is,
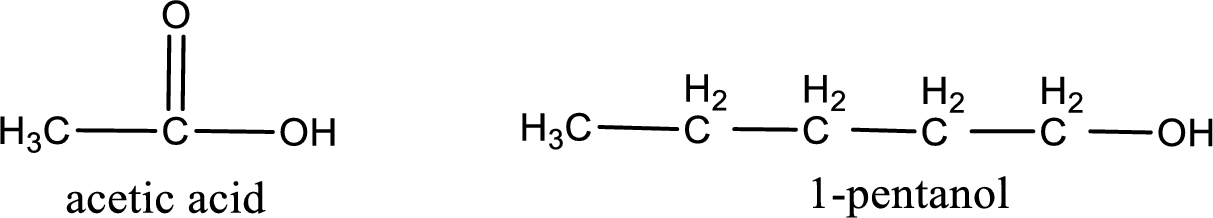
The reaction between two compounds that are shown above, result in the formation of ester. The structure of the ester formed and the complete reaction can be given as shown below,

The structure of ester is,
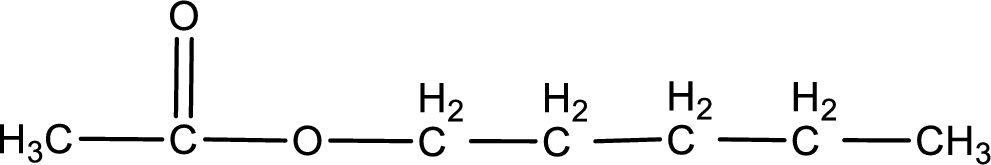
The alkyl and acyl group is identified as shown below,
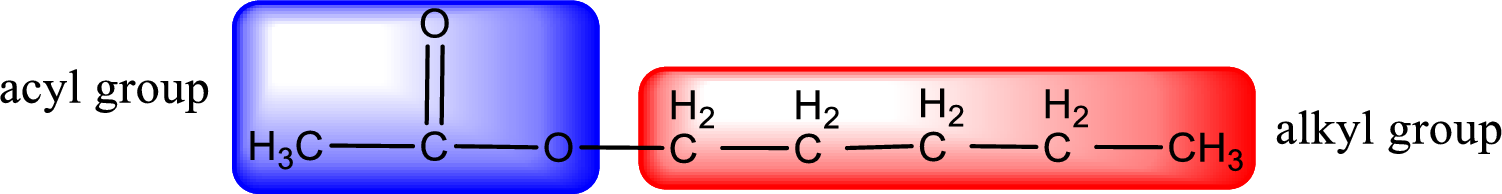
Alkyl group contains five carbon atoms. Hence alkyl group is named as pentyl. The acyl group contains two carbon atoms. The IUPAC name of carboxylic acid that contains three carbon atoms is ethanoic acid. For naming acyl group in ester the suffix “-ic acid” is converted into “-ate”. This gives the name of acyl part as ethanoate. Therefore IUPAC name of the given ester is pentyl ethanoate.
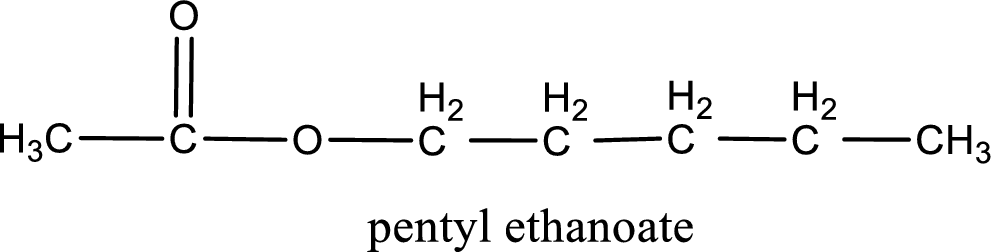
IUPAC name of the formed ester is assigned.
(c)
Interpretation:
The IUPAC name for the ester formed when acetic acid and 2-pentanol react has to be assigned.
Concept Introduction:
Esters are prepared by condensation of carboxylic acid with an alcohol. A molecule of water is lost on this reaction. The reaction that takes place in producing esters is known as esterification reaction.
Esterification reaction is the one in which the carboxylic acid is condensed with an alcohol (or phenol) in presence of strong acid catalyst to produce ester. The general reaction scheme can be given as,
For naming an ester, it can be structurally viewed in a way that contains an acyl group and an alkyl group.
Rules to obtain IUPAC name and common name for an ester:
- Alkyl part appears first in the IUPAC name and it is followed by the acyl part of ester as a separate word.
- Name of the alkyl part in the ester is just a name of R group. It can be alkyl, cycloalkyl, or aryl group.
- Acyl part present in the ester is named by considering the acid name and replacing the suffix “-ic acid” with “-ate”.
- To obtain the common name the alkyl part name is the same while the acyl part name is derived from the common name of the acid by replacing the suffix “-ic acid” with “-ate”.
(c)
Answer to Problem 5.98EP
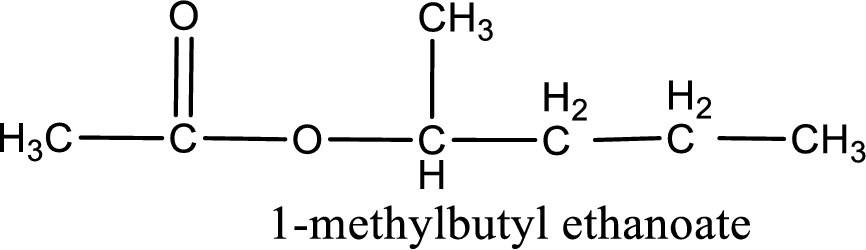
IUPAC name of the ester formed is 1-methylbutyl ethanoate.
Explanation of Solution
Given carboxylic acid and alcohol structure is,
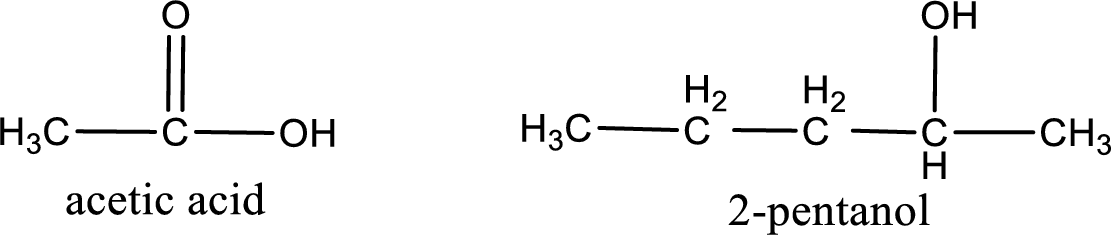
The reaction between two compounds that are shown above, result in the formation of ester. The structure of the ester formed and the complete reaction can be given as shown below,

The structure of ester is,
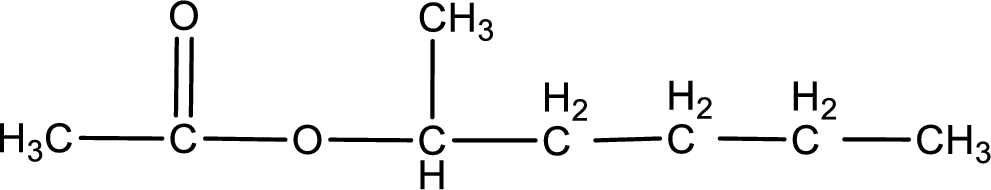
The alkyl and acyl group is identified as shown below,
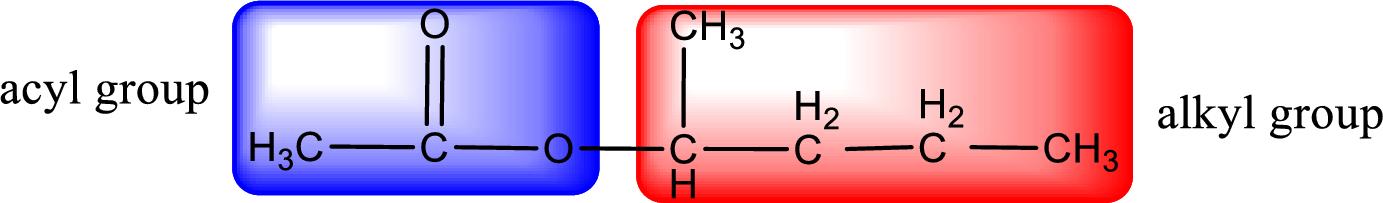
Alkyl group contains five carbon atoms. Four carbon in a long chain with a methyl group substituted in the first carbon atom. Hence alkyl group is named as 1-methylbutyl. The acyl group contains two carbon atoms. The IUPAC name of carboxylic acid that contains three carbon atoms is ethanoic acid. For naming acyl group in ester the suffix “-ic acid” is converted into “-ate”. This gives the name of acyl part as ethanoate. Therefore IUPAC name of the given ester is 1-methylbutyl ethanoate.
IUPAC name of the formed ester is assigned.
(d)
Interpretation:
The IUPAC name for the ester formed when ethanol and benzoic acid react has to be assigned.
Concept Introduction:
Esters are prepared by condensation of carboxylic acid with an alcohol. A molecule of water is lost on this reaction. The reaction that takes place in producing esters is known as esterification reaction.
Esterification reaction is the one in which the carboxylic acid is condensed with an alcohol (or phenol) in presence of strong acid catalyst to produce ester. The general reaction scheme can be given as,
For naming an ester, it can be structurally viewed in a way that contains an acyl group and an alkyl group.
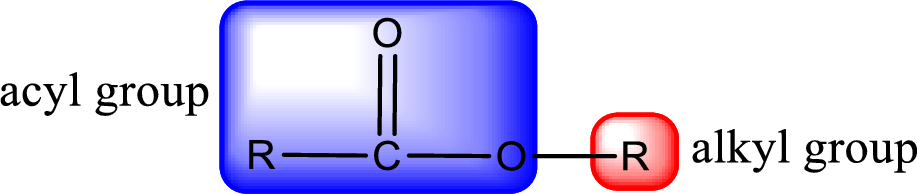
Rules to obtain IUPAC name and common name for an ester:
- Alkyl part appears first in the IUPAC name and it is followed by the acyl part of ester as a separate word.
- Name of the alkyl part in the ester is just a name of R group. It can be alkyl, cycloalkyl, or aryl group.
- Acyl part present in the ester is named by considering the acid name and replacing the suffix “-ic acid” with “-ate”.
- To obtain the common name the alkyl part name is the same while the acyl part name is derived from the common name of the acid by replacing the suffix “-ic acid” with “-ate”.
(d)
Answer to Problem 5.98EP
IUPAC name of the ester formed is ethyl benzoate.
Explanation of Solution
Given carboxylic acid and alcohol structure is,
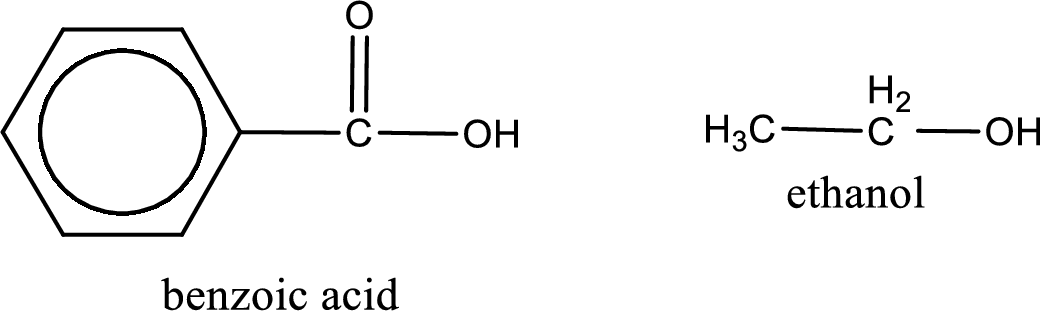
The reaction between two compounds that are shown above, result in the formation of ester. The structure of the ester formed and the complete reaction can be given as shown below,
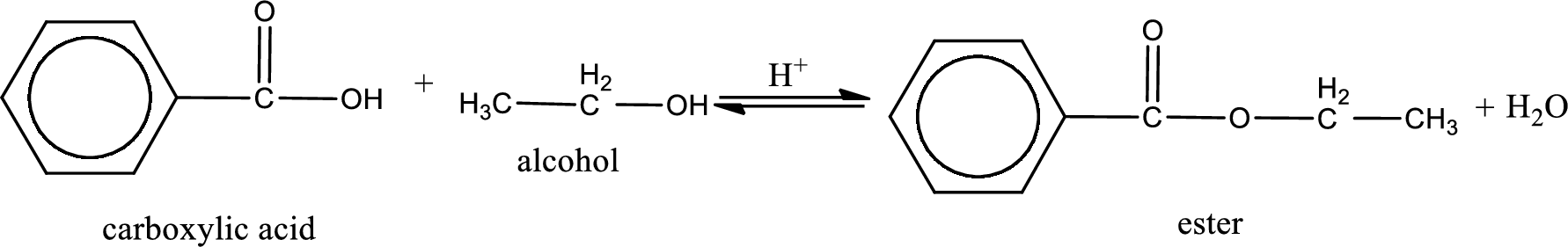
The structure of ester is,
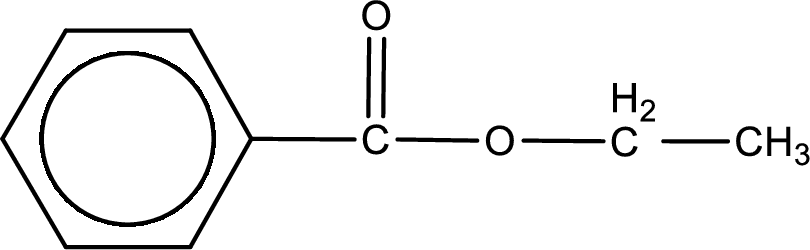
The alkyl and acyl group is identified as shown below,
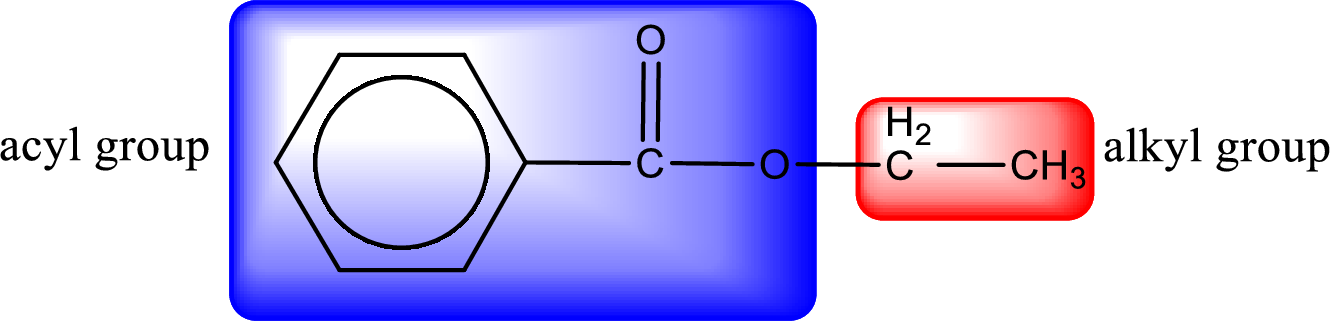
Alkyl group contains only two carbon atoms. Hence alkyl group is named as ethyl. The acyl group contains a benzene ring structure. The IUPAC name of carboxylic acid that contains benzene ring is benzoic acid. For naming acyl group in ester the suffix “-ic acid” is converted into “-ate”. This gives the name of acyl part as benzoate. Therefore IUPAC name of the given ester is ethyl benzoate.
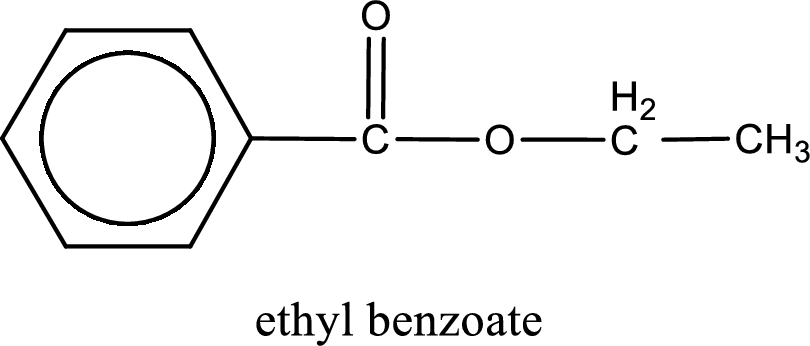
IUPAC name of the formed ester is assigned.
Want to see more full solutions like this?
Chapter 5 Solutions
EBK ORGANIC AND BIOLOGICAL CHEMISTRY
- I'm having trouble with converting lewis diagrams into VSEPR diagrams. I currently have this example of C2BrCl3 which I want to turn into a lewis structure, but I'm not sure what steps I need to do in order to do so. I have the table written down, however, there's two central atoms so what would I do? There seems to be 4 electron domains on the carbon atom and no lone pairs so it would seem like this shape would be tetrahedral. Here's what I have now. Thanks!arrow_forwardWe discussed the solid phase resin using in peptide synthesis. Provide a mechanism, for its formation. DRAW THE MECHANISM.arrow_forwardPlease help. Every time I've asked an expert in the past, it's been wrong :(arrow_forward
- Please help everysingle time ive asked in the past, the solution has been wrongarrow_forwardPlease helparrow_forward(a) 21.8 Name the following compounds. & (b) Br (e) O₂N. (h) H (c) Br (d) NH2 ☑N Br H ہیں Ph (g) OMe бл .0-0.e 21.9 Draw a structural formula for each compound. (a) 2,3-Dinitrotoluene (c) Diphenylmethanol (e) p-Nitroaniline (b) 3-Propylanisole (d) m-Propylphenol (f) Pentabromobenzenearrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





