
Concept explainers
a.
Interpretation:
The direction of the dipole should be indicated in 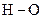 polar covalent bond.
polar covalent bond.
Concept Introduction:
The covalent bond is a type of bond which formed by sharing of electrons between atoms. It forms between two non-metals.
A polar covalent bond is a type of bond where the bonded electrons of covalent bond are shared unequally.
A non-polar covalent bond is a type of bond where the bonded electrons of covalent bond are shared equally.
The relation between elements electronegativity and bonding type are as follows:
| Electro-negativity difference | Bonding type |
| < 0.5 | Non-polar covalent |
| 0.5-2.0 | Polar covalent |
| > 2.0 | Ionic |
The direction of dipole is always from less electronegative atom to more electronegative atom, which indicates the shifting of bonding electrons.
b.
Interpretation:
The direction of the dipole should be indicated in 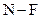 polar covalent bond.
polar covalent bond.
Concept Introduction:
The covalent bond is a type of bond which formed by sharing of electrons between atoms. It forms between two non-metals.
A polar covalent bond is a type of bond where the bonded electrons of covalent bond are shared unequally.
A non-polar covalent bond is a type of bond where the bonded electrons of covalent bond are shared equally.
The relation between elements electronegativity and bonding type are as follows:
| Electro-negativity difference | Bonding type |
| < 0.5 | Non-polar covalent |
| 0.5-2.0 | Polar covalent |
| > 2.0 | Ionic |
The direction of dipole is always from less electronegative atom to more electronegative atom, which indicates the shifting of bonding electrons.
c.
Interpretation:
The direction of the dipole should be indicated in 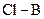 polar covalent bond.
polar covalent bond.
Concept Introduction:
The covalent bond is a type of bond which formed by sharing of electrons between atoms. It forms between two non-metals.
A polar covalent bond is a type of bond where the bonded electrons of covalent bond are shared unequally.
A non-polar covalent bond is a type of bond where the bonded electrons of covalent bond are shared equally.
The relation between elements electronegativity and bonding type are as follows:
| Electro-negativity difference | Bonding type |
| < 0.5 | Non-polar covalent |
| 0.5-2.0 | Polar covalent |
| > 2.0 | Ionic |
The direction of dipole is always from less electronegative atom to more electronegative atom, which indicates the shifting of bonding electrons.
Want to see the full answer?
Check out a sample textbook solution
Chapter 4 Solutions
EBK CHEMISTRY FOR CHANGING TIMES
- Match the denticity to the ligand. Water monodentate ✓ C₂O2 bidentate H₂NCH₂NHCH2NH2 bidentate x EDTA hexadentate Question 12 Partially correct Mark 2 out of 2 Flag question Provide the required information for the coordination compound shown below: Na NC-Ag-CN] Number of ligands: 20 Coordination number: 2✔ Geometry: linear Oxidation state of transition metal ion: +3 x in 12 correct out of 2 question Provide the required information for the coordination compound shown below. Na NC-Ag-CN] Number of ligands: 20 Coordination number: 2 Geometry: linear 0 Oxidation state of transition metal ion: +3Xarrow_forwardCan you explain step by step behind what the synthetic strategy would be?arrow_forwardPlease explain step by step in detail the reasoning behind this problem/approach/and answer. thank you!arrow_forward
- 2. Predict the product(s) that forms and explain why it forms. Assume that any necessary catalytic acid is present. .OH HO H₂N OHarrow_forwardconsider the rate of the reaction below to be r. Whats the rate after each reaction? Br + NaCN CN + NaBr a. Double the concentration of alkyl bromide b. Halve the concentration of the electrophile & triple concentration of cyanide c. Halve the concentration of alkyl chloridearrow_forwardPredict the organic reactant that is involved in the reaction below, and draw the skeletal ("line") structures of the missing organic reactant. Please include all steps & drawings & explanations.arrow_forward
- What are the missing reagents for the spots labeled 1 and 3? Please give a detailed explanation and include the drawings and show how the synthesis proceeds with the reagents.arrow_forwardWhat is the organic molecule X of the following acetal hydrolysis? Please draw a skeletal line structure and include a detailed explanation and drawing of how the mechanism proceeds. Please include any relevant information that is needed to understand the process of acetal hydrolysis.arrow_forwardWhat are is the organic molecule X and product Y of the following acetal hydrolysis? Please draw a skeletal line structure and include a detailed explanation and drawing of how the mechanism proceeds. Please include any relevant information that is needed to understand the process of acetal hydrolysis.arrow_forward
- At 300 K, in the decomposition reaction of a reactant R into products, several measurements of the concentration of R over time have been made (see table). Without using graphs, calculate the order of the reaction. t/s [R]/(mol L-1) 0 0,5 171 0,16 720 0,05 1400 0,027arrow_forwardPredict the organic products that form in the reaction below, and draw the skeletal ("line") structures of the missing organic products. Please include all steps & drawings & explanations.arrow_forwardWhat are the missing reagents for the spots labeled 1 and 3? Please give a detailed explanation and include the drawings and show how the synthesis proceeds with the reagents.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





