
(a)
Interpretation:
The structure of predominant organic product that is formed in the given reaction has to be drawn.
Concept Introduction:
Alcohols undergo halogenation reaction to give halogenated product. Alcohols on halogenation gives halogenated product in which the hydroxyl group present in the alcohol is substituted by the halogen. Phosphorous trihalides are useful in producing
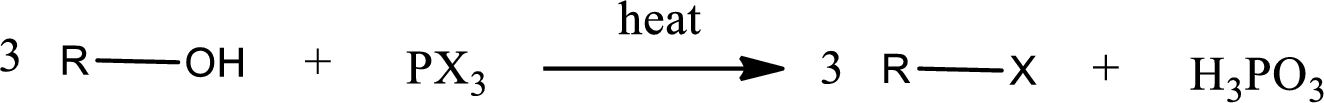
(b)
Interpretation:
The structure of predominant organic product that is formed in the given reaction has to be drawn.
Concept Introduction:
Dehydration reaction is the loss of water from a single reactant. Alcohol undergoes dehydration reaction to form
(c)
Interpretation:
The structure of predominant organic product that is formed in the given reaction has to be drawn.
Concept Introduction:
In
In organic chemistry, reduction reaction is referred to the number
Alcohols do undergo
(d)
Interpretation:
The structure of predominant organic product that is formed in the given reaction has to be drawn.
Concept Introduction:
Dehydration reaction is the loss of water from a single reactant. Alcohol undergoes dehydration reaction to form alkene. Sulfuric acid acts as a catalyst for hydration of alkene at room temperature. The same sulfuric acid acts as a dehydrating agent when treated with alcohol at high temperature. If the reaction is carried out at a lower temperature, the loss of water molecule takes place from two molecule of reactant. This results in the formation of ether. Primary alcohol when treated with sulfuric acid at lower temperature (
Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
Organic And Biological Chemistry
- What is the final product when hexanedioic acid reacts with 1º PCl5 and 2º NH3.arrow_forwardWhat is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forward
- The temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning





