
Concept explainers
(a)
Interpretation:
The IUPAC name and common name for the given thioether has to be assigned.
Concept Introduction:
Any organic molecule can be named by using certain rules given by IUPAC (International Union for Pure and applied chemistry). IUPAC name consists of three parts in major namely Prefix suffix and root word.
Prefix represents the substituent present in the molecule and its position in the root name.
Suffix denotes the presence of
Root word represents the longest continuous carbon skeleton of the organic molecule.
IUPAC rules for naming thioether:
- ✓ The base name is found from the longest carbon chain present in thioether.
- ✓ The suffix –thio has to be added in order to obtain the alkylthio group name. For example, ethyl becomes as ethylthio, methyl becomes as methylthio etc.
- ✓ Alkylthio name has to be placed first with the number (carbon atom to which the alkykthio group is attached) followed by the base name.
Rules for assigning common names to thioether:
For obtaining common name for thioether, two rules are applicable, one for symmetrical ethers and one for unsymmetrical ethers.
- ✓ For unsymmetrical thioethers, the two hydrocarbon groups that is attached to the oxygen atom is arranged in an alphabetical order and the word sulfide is added. The words are separated by a space. These names have three words with space between them.
- ✓ For symmetrical ethers, prefix di- is used. Then the word sulfide is added with a space between the two words. These names have two words with space between them.
(a)
Answer to Problem 3.143EP
IUPAC name for the given compound is methylthioethane and common name is ethyl methyl sulfide.
Explanation of Solution
Given structure of compound is shown below,
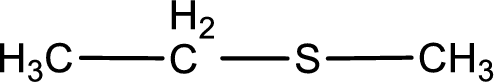
First step is to identify the longest carbon chain. In this case it is a two carbon chain. Hence, the base name is ethane.
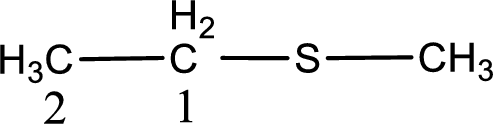
Next step is to identify the alkylthio group. In the given thioether, the alkylthio group is found to be methylthio as it contains only one carbon atom.
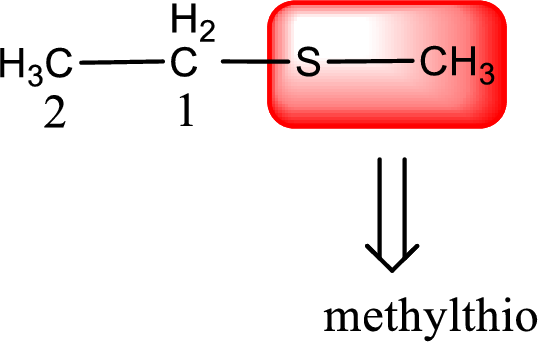
Alkylthio name is placed before the base name with the appropriate number that gives information about to which carbon atom the alkylthio group is attached. This gives the IUPAC name as methylthioethane.
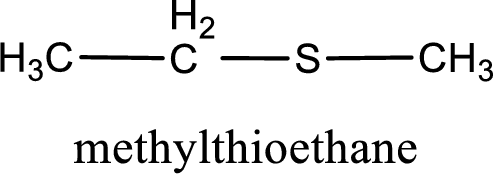
The IUPAC name of the given thioether is methylthioethane.
To obtain common name the two hydrocarbon groups that are attached to the sulfur atom is named first. In the given structure, a methyl and an ethyl group is present. Arranging them in the alphabetical order and adding the word sulfide after them gives the common name for the given thioether. Common name for the given thioether is ethyl methyl sulfide.
IUPAC name and common name for the given thioether is assigned.
(b)
Interpretation:
The IUPAC name and common name for the given thioether has to be assigned.
Concept Introduction:
Any organic molecule can be named by using certain rules given by IUPAC (International Union for Pure and applied chemistry). IUPAC name consists of three parts in major namely Prefix suffix and root word.
Prefix represents the substituent present in the molecule and its position in the root name.
Suffix denotes the presence of functional group if any in the molecule. It can be an alkene, alkyne, alcohol, carboxylic acid, alcohol etc.
Root word represents the longest continuous carbon skeleton of the organic molecule.
IUPAC rules for naming thioether:
- ✓ The base name is found from the longest carbon chain present in thioether.
- ✓ The suffix –thio has to be added in order to obtain the alkylthio group name. For example, ethyl becomes as ethylthio, methyl becomes as methylthio etc.
- ✓ Alkylthio name has to be placed first with the number (carbon atom to which the alkykthio group is attached) followed by the base name.
Rules for assigning common names to thioether:
For obtaining common name for thioether, two rules are applicable, one for symmetrical ethers and one for unsymmetrical ethers.
- ✓ For unsymmetrical thioethers, the two hydrocarbon groups that is attached to the oxygen atom is arranged in an alphabetical order and the word sulfide is added. The words are separated by a space. These names have three words with space between them.
- ✓ For symmetrical ethers, prefix di- is used. Then the word sulfide is added with a space between the two words. These names have two words with space between them.
(b)
Answer to Problem 3.143EP
IUPAC name for the given compound is 2-methylthiopropane and common name is isopropyl methyl sulfide.
Explanation of Solution
Given structure of compound is shown below,
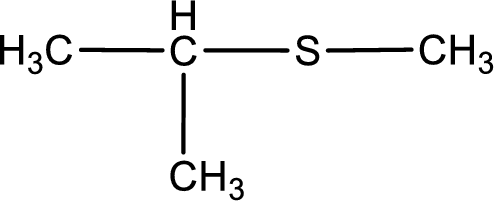
First step is to identify the longest carbon chain. In this case it is a three carbon chain. Hence, the base name is propane.
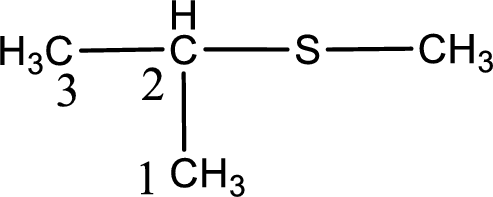
Next step is to identify the alkylthio group. In the given thioether, the alkylthio group is found to be methylthio as it contains only one carbon atom. The point of attachment in the propane for methylthio group is in the second carbon atom.
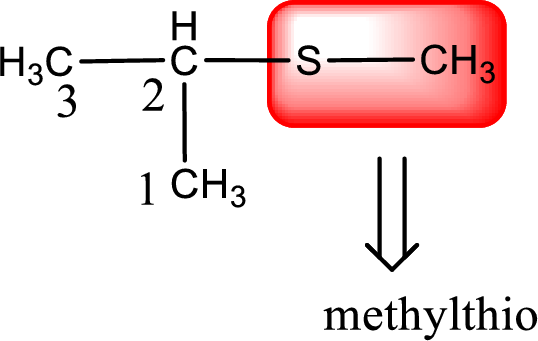
Alkylthio name is placed before the base name with the appropriate number that gives information about to which carbon atom the alkylthio group is attached. This gives the IUPAC name as 2-methylthiopropane.
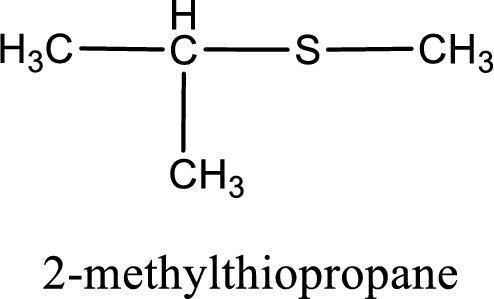
The IUPAC name of the given thioether is 2-methylthiopropane.
To obtain common name the two hydrocarbon groups that are attached to the sulfur atom is named first. In the given structure, a methyl and an isopropyl group is present. Arranging them in the alphabetical order and adding the word sulfide after them gives the common name for the given thioether. Common name for the given thioether is isopropyl methyl sulfide.
IUPAC name and common name for the given thioether is assigned.
(c)
Interpretation:
The IUPAC name and common name for the given thioether has to be assigned.
Concept Introduction:
Any organic molecule can be named by using certain rules given by IUPAC (International Union for Pure and applied chemistry). IUPAC name consists of three parts in major namely Prefix suffix and root word.
Prefix represents the substituent present in the molecule and its position in the root name.
Suffix denotes the presence of functional group if any in the molecule. It can be an alkene, alkyne, alcohol, carboxylic acid, alcohol etc.
Root word represents the longest continuous carbon skeleton of the organic molecule.
IUPAC rules for naming thioether:
- ✓ The base name is found from the longest carbon chain present in thioether.
- ✓ The suffix –thio has to be added in order to obtain the alkylthio group name. For example, ethyl becomes as ethylthio, methyl becomes as methylthio etc.
- ✓ Alkylthio name has to be placed first with the number (carbon atom to which the alkykthio group is attached) followed by the base name.
Rules for assigning common names to thioether:
For obtaining common name for thioether, two rules are applicable, one for symmetrical ethers and one for unsymmetrical ethers.
- ✓ For unsymmetrical thioethers, the two hydrocarbon groups that is attached to the oxygen atom is arranged in an alphabetical order and the word sulfide is added. The words are separated by a space. These names have three words with space between them.
- ✓ For symmetrical ethers, prefix di- is used. Then the word sulfide is added with a space between the two words. These names have two words with space between them.
(c)
Answer to Problem 3.143EP
IUPAC name for the given compound is methylthiocyclohexane and common name is cyclohexyl methyl sulfide.
Explanation of Solution
Given structure of compound is shown below,
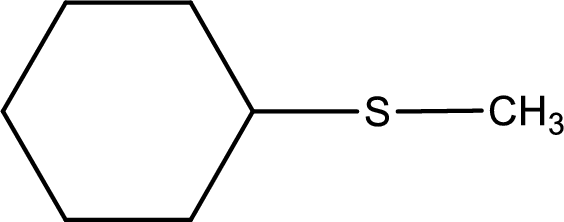
First step is to identify the longest carbon chain. In this case it is a six carbon cyclic chain that is saturated. Hence, the base name is cyclohexane.
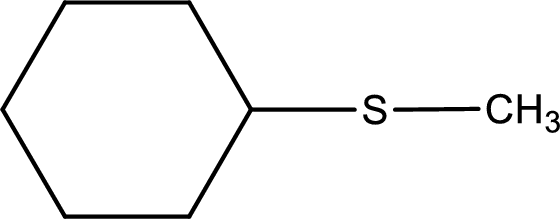
Next step is to identify the alkylthio group. In the given thioether, the alkylthio group is found to be methylthio as it contains only one carbon atom.
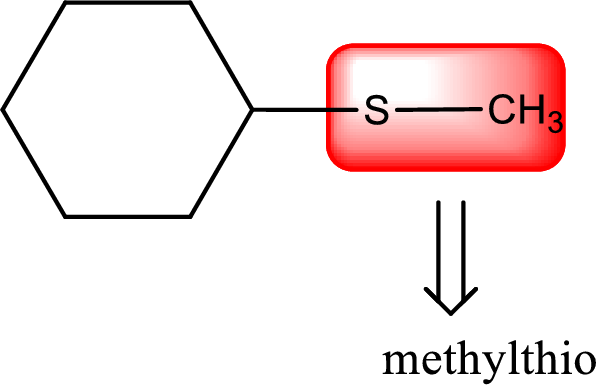
Alkylthio name is placed before the base name with the appropriate number that gives information about to which carbon atom the alkylthio group is attached. This gives the IUPAC name as methylthiocyclohexane.
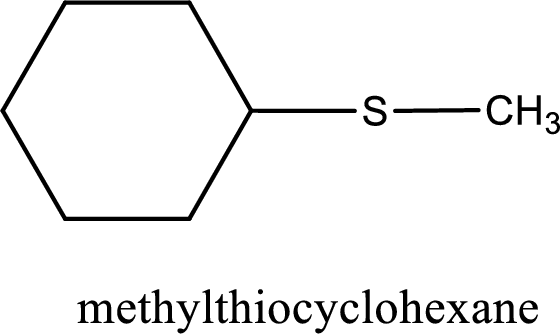
The IUPAC name of the given thioether is methylthiocyclohexane.
To obtain common name the two hydrocarbon groups that are attached to the sulfur atom is named first. In the given structure, a methyl and a cyclohexyl group is present. Arranging them in the alphabetical order and adding the word sulfide after them gives the common name for the given thioether. Common name for the given thioether is cyclohexyl methyl sulfide.
IUPAC name and common name for the given thioether is assigned.
(d)
Interpretation:
The IUPAC name and common name for the given thioether has to be assigned.
Concept Introduction:
Any organic molecule can be named by using certain rules given by IUPAC (International Union for Pure and applied chemistry). IUPAC name consists of three parts in major namely Prefix suffix and root word.
Prefix represents the substituent present in the molecule and its position in the root name.
Suffix denotes the presence of functional group if any in the molecule. It can be an alkene, alkyne, alcohol, carboxylic acid, alcohol etc.
Root word represents the longest continuous carbon skeleton of the organic molecule.
IUPAC rules for naming thioether:
- ✓ The base name is found from the longest carbon chain present in thioether.
- ✓ The suffix –thio has to be added in order to obtain the alkylthio group name. For example, ethyl becomes as ethylthio, methyl becomes as methylthio etc.
- ✓ Alkylthio name has to be placed first with the number (carbon atom to which the alkykthio group is attached) followed by the base name.
Rules for assigning common names to thioether:
For obtaining common name for thioether, two rules are applicable, one for symmetrical ethers and one for unsymmetrical ethers.
- ✓ For unsymmetrical thioethers, the two hydrocarbon groups that is attached to the oxygen atom is arranged in an alphabetical order and the word sulfide is added. The words are separated by a space. These names have three words with space between them.
- ✓ For symmetrical ethers, prefix di- is used. Then the word sulfide is added with a space between the two words. These names have two words with space between them.
(d)
Answer to Problem 3.143EP
IUPAC name for the given compound is 3-(methylthio)-1-propene and common name is allyl methyl sulfide.
Explanation of Solution
Given structure of compound is shown below,
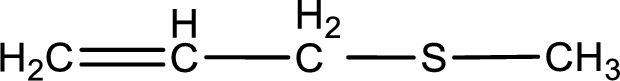
First step is to identify the longest carbon chain. In this case it is a three carbon chain with a double bond. Hence, the base name is propene.
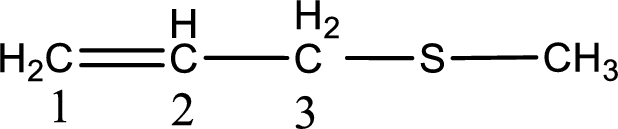
Next step is to identify the alkylthio group. In the given thioether, the alkylthio group is found to be methylthio as it contains only one carbon atom.
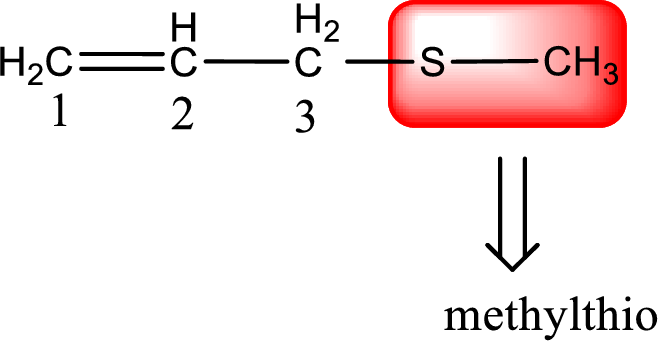
Alkylthio name is placed before the base name with the appropriate number that gives information about to which carbon atom the alkylthio group is attached. This gives the IUPAC name as 3-(methylthio)-1-propene.
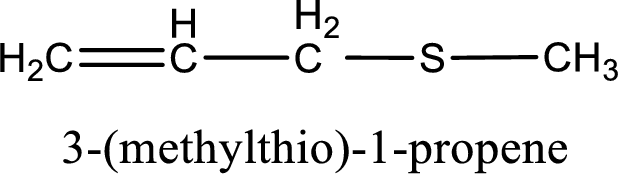
The IUPAC name of the given thioether is 3-(methylthio)-1-propene.
To obtain common name the two hydrocarbon groups that are attached to the sulfur atom is named first. In the given structure, a methyl and an allyl group is present. Arranging them in the alphabetical order and adding the word sulfide after them gives the common name for the given thioether. Common name for the given thioether is allyl methyl sulfide.
IUPAC name and common name for the given thioether is assigned.
Want to see more full solutions like this?
Chapter 3 Solutions
Organic And Biological Chemistry
- The following equations represent the formation of compound MX. What is the AH for the electron affinity of X (g)? X₂ (g) → 2X (g) M (s) → M (g) M (g) M (g) + e- AH = 60 kJ/mol AH = 22 kJ/mol X (g) + e-X (g) M* (g) +X (g) → MX (s) AH = 118 kJ/mol AH = ? AH = -190 kJ/mol AH = -100 kJ/mol a) -80 kJ b) -30 kJ c) -20 kJ d) 20 kJ e) 156 kJarrow_forwardA covalent bond is the result of the a) b) c) d) e) overlap of two half-filled s orbitals overlap of a half-filled s orbital and a half-filled p orbital overlap of two half-filled p orbitals along their axes parallel overlap of two half-filled parallel p orbitals all of the abovearrow_forwardCan the target compound at right be efficiently synthesized in good yield from the unsubstituted benzene at left? starting material target If so, draw a synthesis below. If no synthesis using reagents ALEKS recognizes is possible, check the box under the drawing area. Be sure you follow the standard ALEKS rules for submitting syntheses. + More... Note for advanced students: you may assume that you are using a large excess of benzene as your starting material. C T Add/Remove step X ноarrow_forward
- Which one of the following atoms should have the largest electron affinity? a) b) c) d) 으으 e) 1s² 2s² 2p6 3s¹ 1s² 2s² 2p5 1s² 2s² 2p 3s² 3p² 1s² 2s 2p 3s² 3p6 4s2 3ds 1s² 2s² 2p6arrow_forwardAll of the following are allowed energy levels except _. a) 3f b) 1s c) 3d d) 5p e) 6sarrow_forwardA student wants to make the following product in good yield from a single transformation step, starting from benzene. Add any organic reagents the student is missing on the left-hand side of the arrow, and any addition reagents that are necessary above or below the arrow. If this product can't be made in good yield with a single transformation step, check the box below the drawing area. Note for advanced students: you may assume that an excess of benzene is used as part of the reaction conditions. : ☐ + I X This product can't be made in a single transformation step.arrow_forward
- Ppplllleeeaaasssseeee helllppp wiithhh thisss Organic chemistryyyyyy I talked like this because AI is very annoyingarrow_forwardName the family to which each organic compound belongs. The first answer has been filled in for you. compound CH₂ || CH3-C-NH2 0 ။ CH3-C-CH₂ CH=O–CH=CH, CH₂ HO CH2-CH2-CH-CH3 family amine Darrow_forward1b. Br LOHarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning




