
(a)
Interpretation:
The structure of predominant organic product that is formed in the given reaction has to be drawn.
Concept Introduction:
Alcohols undergo halogenation reaction to give halogenated product. Alcohols on halogenation gives halogenated product in which the hydroxyl group present in the alcohol is substituted by the halogen. Phosphorous trihalides are useful in producing
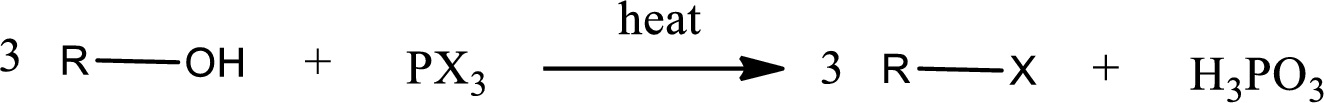
(b)
Interpretation:
The structure of predominant organic product that is formed in the given reaction has to be drawn.
Concept Introduction:
Dehydration reaction is the loss of water from a single reactant. Alcohol undergoes dehydration reaction to form
(c)
Interpretation:
The structure of predominant organic product that is formed in the given reaction has to be drawn.
Concept Introduction:
In
In organic chemistry, reduction reaction is referred to the number
Alcohols do undergo
(d)
Interpretation:
The structure of predominant organic product that is formed in the given reaction has to be drawn.
Concept Introduction:
Dehydration reaction is the loss of water from a single reactant. Alcohol undergoes dehydration reaction to form alkene. Sulfuric acid acts as a catalyst for hydration of alkene at room temperature. The same sulfuric acid acts as a dehydrating agent when treated with alcohol at high temperature. If the reaction is carried out at a lower temperature, the loss of water molecule takes place from two molecule of reactant. This results in the formation of ether. Primary alcohol when treated with sulfuric acid at lower temperature (
Trending nowThis is a popular solution!

Chapter 3 Solutions
Organic And Biological Chemistry
- Indicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forwardIn the two chair conformations of glucose, the most stable is the one with all the OH groups in the equatorial position. Is this correct?arrow_forwardIndicate the formula of the product obtained by reacting D-Galactose with hydroxylamine.arrow_forward
- helparrow_forwardThe temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning





