
Physics: Principles with Applications
6th Edition
ISBN: 9780130606204
Author: Douglas C. Giancoli
Publisher: Prentice Hall
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 23, Problem 50P
To determine
The proof using the ray diagram that a real image formed by a thin lens is always inverted, where as a virtual image is always upright if the object is real.
Expert Solution & Answer
Answer to Problem 50P
Solution:
If image is real and inverted.
If image is inverted and erect.
Explanation of Solution
If image is real and inverted.
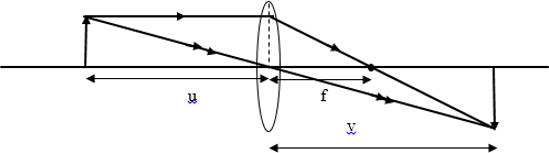
If image is inverted and erect.
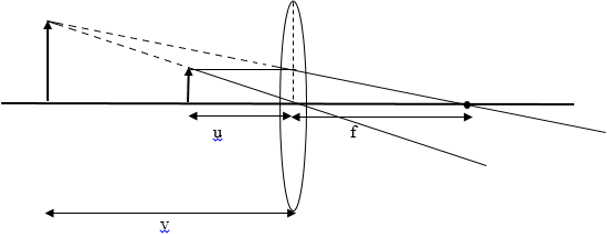
If image is inverted and erect.
Chapter 23 Solutions
Physics: Principles with Applications
Ch. 23 - Prob. 1QCh. 23 - What is the focal length of a plane mirror? What...Ch. 23 - Prob. 3QCh. 23 - Prob. 4QCh. 23 - Prob. 5QCh. 23 - Prob. 6QCh. 23 - Prob. 7QCh. 23 - Prob. 8QCh. 23 - Prob. 9QCh. 23 - Prob. 10Q
Ch. 23 - Prob. 11QCh. 23 - You look into an aquarium and view a fish inside....Ch. 23 - Prob. 13QCh. 23 - Prob. 14QCh. 23 - A child looks into a pool to see how deep it is....Ch. 23 - Prob. 16QCh. 23 - Prob. 17QCh. 23 - Prob. 18QCh. 23 - Prob. 19QCh. 23 - Prob. 20QCh. 23 - Prob. 21QCh. 23 - Prob. 22QCh. 23 - Prob. 23QCh. 23 - Prob. 24QCh. 23 - Prob. 25QCh. 23 - Prob. 26QCh. 23 - Prob. 27QCh. 23 - Prob. 28QCh. 23 - Prob. 29QCh. 23 - Prob. 30QCh. 23 - Prob. 31QCh. 23 - Prob. 32QCh. 23 - Prob. 33QCh. 23 - Prob. 1PCh. 23 - Prob. 2PCh. 23 - Two plane mirrors meet at a 1350 angle, Fig....Ch. 23 - Prob. 4PCh. 23 - Prob. 5PCh. 23 - Prob. 6PCh. 23 - Suppose you are 94 cm from a plane mirror. What...Ch. 23 - A solar cooker, really a concave mirror pointed at...Ch. 23 - How far from a concave mirror (radius 21.0 cm)...Ch. 23 - A small candle is 38 cm from a concave mirror...Ch. 23 - An object 3.0 mm high is placed 16 cm from a...Ch. 23 - A dentist wants a small mirror that, when 2.00 cm...Ch. 23 - You are standing 3.4 m from a convex security...Ch. 23 - The image of a distant tree is virtual and very...Ch. 23 - Prob. 15PCh. 23 - Prob. 16PCh. 23 - Prob. 17PCh. 23 - Some rearview mirrors produce images of cars to...Ch. 23 - Prob. 19PCh. 23 - Prob. 20PCh. 23 - Prob. 21PCh. 23 - Prob. 22PCh. 23 - Prob. 23PCh. 23 - Prob. 24PCh. 23 - Prob. 25PCh. 23 - Prob. 26PCh. 23 - Prob. 27PCh. 23 - Prob. 28PCh. 23 - Prob. 29PCh. 23 - Prob. 30PCh. 23 - Prob. 31PCh. 23 - Rays of the Sunare seen to make a 36.0° angle to...Ch. 23 - Prob. 33PCh. 23 - A beam of light in air strikes a slab of glass (n...Ch. 23 - Prob. 35PCh. 23 - Prob. 36PCh. 23 - Prob. 37PCh. 23 - Prob. 38PCh. 23 - Prob. 39PCh. 23 - Prob. 40PCh. 23 - 39. (Ill) (a) What is the minimum index of...Ch. 23 - 40. (Ill) A beam of light enters the end of an...Ch. 23 - Prob. 43PCh. 23 - Prob. 44PCh. 23 - Prob. 45PCh. 23 - Prob. 46PCh. 23 - Prob. 47PCh. 23 - Prob. 48PCh. 23 - Prob. 49PCh. 23 - Prob. 50PCh. 23 - A stamp collector uses a converging lens with...Ch. 23 - Prob. 52PCh. 23 - Prob. 53PCh. 23 - Prob. 54PCh. 23 - Prob. 55PCh. 23 - Prob. 56PCh. 23 - Prob. 57PCh. 23 - Prob. 58PCh. 23 - Prob. 59PCh. 23 - Prob. 60PCh. 23 - A diverging lens with f= -36.5 cm is placed 14.0...Ch. 23 - Prob. 62PCh. 23 - Prob. 63PCh. 23 - Two lenses, one converging with focal length 20.0...Ch. 23 - Prob. 65PCh. 23 - A double concave lens has surface radii of 33.4 cm...Ch. 23 - Prob. 67PCh. 23 - Prob. 68PCh. 23 - Prob. 69PCh. 23 - Prob. 70PCh. 23 - Prob. 71PCh. 23 - Prob. 72GPCh. 23 - Prob. 73GPCh. 23 - Prob. 74GPCh. 23 - The critical angle of a certain piece of plastic...Ch. 23 - Prob. 76GPCh. 23 - Prob. 77GPCh. 23 - Prob. 78GPCh. 23 - Prob. 79GPCh. 23 - Prob. 80GPCh. 23 - 77
77. If the apex of a prism is ? = 75o (see...Ch. 23 - Prob. 82GPCh. 23 - Prob. 83GPCh. 23 - Prob. 84GPCh. 23 - Prob. 85GPCh. 23 - Prob. 86GPCh. 23 - Prob. 87GPCh. 23 - Figure 23-65is a photograph of an eyeball with the...Ch. 23 - Prob. 89GPCh. 23 - Prob. 90GPCh. 23 - 87 ‘(a) Show that if two thin lenses of focal...Ch. 23 - Prob. 92GPCh. 23 - Prob. 93GPCh. 23 - Prob. 94GP
Additional Science Textbook Solutions
Find more solutions based on key concepts
SCIENTIFIC INQUIRY You are handed a mystery pea plant with tall stems and axial flowers and asked to determine ...
Campbell Biology (11th Edition)
One isomer of methamphetamine is the addictive illegal drug known as crank. Another isomer is a medicine for si...
Campbell Essential Biology (7th Edition)
29. For the reaction
determine the expression for the rate of the reaction in terms of the change in concentr...
Chemistry: Structure and Properties (2nd Edition)
Heat lamps are commonly used to maintain foods at about 50C for as long as 12 hours in cafeteria serving lines....
Microbiology: An Introduction
2. Define equilibrium population. Outline the conditions that must be met for a population to stay in genetic e...
Biology: Life on Earth (11th Edition)
How Would the experiments result charge if oxygen (O2) were induced in the spark chamber?
Biology: Life on Earth with Physiology (11th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- 4) Consider the pulley (Mass = 20kg, Radius 0.3m) shown in the picture. Model this pulley as a uniform solid disk (1 = (1/2) MR2) that is hinged at its center of mass. If the hanging mass is 30 kg, and is released, (a) compute the angular acceleration of the pulley (b) calculate the acceleration of the hanging mass. A o 0.3 3019 20KSarrow_forwardRefer to the image attachedarrow_forwardShrinking Loop. A circular loop of flexible iron wire has an initial circumference of 161 cm , but its circumference is decreasing at a constant rate of 15.0 cm/s due to a tangential pull on the wire. The loop is in a constant uniform magnetic field of magnitude 1.00 T , which is oriented perpendicular to the plane of the loop. Assume that you are facing the loop and that the magnetic field points into the loop. Find the magnitude of the emf E induced in the loop after exactly time 9.00 s has passed since the circumference of the loop started to decrease. Find the direction of the induced current in the loop as viewed looking along the direction of the magnetic field. Please explain all stepsarrow_forward
- Make up an application physics principle problem that provides three (3) significant equations based on the concepts of capacitors and ohm's law.arrow_forwardA straight horizontal garden hose 38.0 m long with an interior diameter of 1.50 cm is used to deliver 20oC water at the rate of 0.590 liters/s. Assuming that Poiseuille's Law applies, estimate the pressure drop (in Pa) from one end of the hose to the other.arrow_forwardA rectangle measuring 30.0 cm by 40.0 cm is located inside a region of a spatially uniform magnetic field of 1.70 T , with the field perpendicular to the plane of the coil (the figure (Figure 1)). The coil is pulled out at a steady rate of 2.00 cm/s traveling perpendicular to the field lines. The region of the field ends abruptly as shown. Find the emf induced in this coil when it is all inside the field, when it is partly in the field, and when it is fully outside. Please show all steps.arrow_forward
- A rectangular circuit is moved at a constant velocity of 3.00 m/s into, through, and then out of a uniform 1.25 T magnetic field, as shown in the figure (Figure 1). The magnetic field region is considerably wider than 50.0 cm . Find the direction (clockwise or counterclockwise) of the current induced in the circuit as it is going into the magnetic field (the first case), totally within the magnetic field but still moving (the second case), and moving out of the field (the third case). Find the magnitude of the current induced in the circuit as it is going into the magnetic field . Find the magnitude of the current induced in the circuit as it is totally within the magnetic field but still moving. Find the magnitude of the current induced in the circuit as it is moving out of the field. Please show all stepsarrow_forwardShrinking Loop. A circular loop of flexible iron wire has an initial circumference of 161 cm , but its circumference is decreasing at a constant rate of 15.0 cm/s due to a tangential pull on the wire. The loop is in a constant uniform magnetic field of magnitude 1.00 T , which is oriented perpendicular to the plane of the loop. Assume that you are facing the loop and that the magnetic field points into the loop. Find the magnitude of the emf E induced in the loop after exactly time 9.00 s has passed since the circumference of the loop started to decrease. Find the direction of the induced current in the loop as viewed looking along the direction of the magnetic field. Please explain all stepsarrow_forwardA circular loop of wire with radius 0.0480 m and resistance 0.163 Ω is in a region of spatially uniform magnetic field, as shown in the following figure (Figure 1). The magnetic field is directed out of the plane of the figure. The magnetic field has an initial value of 7.88 T and is decreasing at a rate of -0.696 T/s . Is the induced current in the loop clockwise or counterclockwise? What is the rate at which electrical energy is being dissipated by the resistance of the loop? Please explain all stepsarrow_forward
- A 0.333 m long metal bar is pulled to the left by an applied force F and moves to the left at a constant speed of 5.90 m/s. The bar rides on parallel metal rails connected through a 46.7 Ω resistor, as shown in (Figure 1), so the apparatus makes a complete circuit. You can ignore the resistance of the bar and rails. The circuit is in a uniform 0.625 T magnetic field that is directed out of the plane of the figure. Is the induced current in the circuit clockwise or counterclockwise? What is the rate at which the applied force is doing work on the bar? Please explain all stepsarrow_forwardA 0.850-m-long metal bar is pulled to the right at a steady 5.0 m/s perpendicular to a uniform, 0.650-T magnetic field. The bar rides on parallel metal rails connected through a 25-Ω, resistor (Figure 1), so the apparatus makes a complete circuit. Ignore the resistance of the bar and the rails. Calculate the magnitude of the emf induced in the circuit. Find the direction of the current induced in the circuit. Calculate the current through the resistor.arrow_forwardIn the figure, a conducting rod with length L = 29.0 cm moves in a magnetic field B→ of magnitude 0.510 T directed into the plane of the figure. The rod moves with speed v = 5.00 m/s in the direction shown. When the charges in the rod are in equilibrium, which point, a or b, has an excess of positive charge and where does the electric field point? What is the magnitude E of the electric field within the rod, the potential difference between the ends of the rod, and the magnitude E of the motional emf induced in the rod? Which point has a higher potential? Please explain all stepsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON
Convex and Concave Lenses; Author: Manocha Academy;https://www.youtube.com/watch?v=CJ6aB5ULqa0;License: Standard YouTube License, CC-BY