
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 21.SE, Problem 80AP
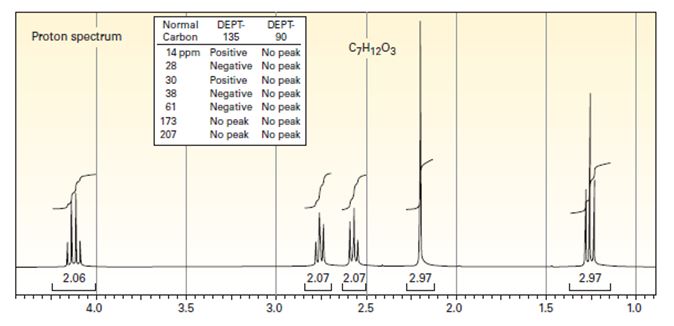
Draw the structure of the compound that produced the spectra below. The infrared spectrum has strong bands at 1720 and 1738 cm-1.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The reaction of a carboxylic acid with an alcohol in the presence of acid is termed Fischer esterification.
OH + CH₂OH
На
B C
A. The nucleophile in this reaction is
B. Compound C functions as
a.
a base scavenger
b.
a solvent
C.
a catalyst
d.
a neutralizer
C. Fischer esterification is an example of: .
a. nucleophilic acyl addition
b. nucleophilic acyl substitution
c. nucleophilic acyl elimination
d. nucleophilic acyl rearrangement
in this reaction.
A highly useful and general method for the synthesis of alcohols is the addition of Grignard reagents to
carbonyl compounds. Show what Grignard reagent and what carbonyl compound you would start with
to prepare each alcohol below. List all possibilities.
OH
C-CH2CH3
CH3
Rank the following groups of compounds from most acidic (1) to least acidic (4). Place the number
corresponding to the compound's relative rank in the blank below the structure.
NO2
a.
b.
NO2
NO2
CH,CH,CH,CH,OH. CHCHCH-CHOH. CH-CH-CH,CH;OH CH-CHCH-CH-OH
OH
OH
CH₂OH
COH
ဒီ ပုံ ပုံ
H&C
CN
CN
ĆN
Chapter 21 Solutions
Organic Chemistry
Ch. 21.1 - Give IUPAC names for the following substances:Ch. 21.1 - Draw structures corresponding to the following...Ch. 21.2 - Prob. 3PCh. 21.2 - Rank the compounds in each of the following sets...Ch. 21.2 - Predict the products of the following nucleophilic...Ch. 21.2 - Prob. 6PCh. 21.3 - Prob. 7PCh. 21.3 - If the following molecule is treated with acid...Ch. 21.4 - How might you prepare the following esters using a...Ch. 21.4 - Prob. 10P
Ch. 21.4 - Prob. 11PCh. 21.4 - Prob. 12PCh. 21.4 - Prob. 13PCh. 21.5 - Prob. 14PCh. 21.5 - What product would you expect from reaction of one...Ch. 21.6 - Prob. 16PCh. 21.6 - Prob. 17PCh. 21.6 - Show the products you would obtain by reduction of...Ch. 21.6 - What ester and what Grignard reagent might you...Ch. 21.7 - Prob. 20PCh. 21.7 - How would you use the reaction of an amide with...Ch. 21.8 - Write the mechanism of the reaction shown in...Ch. 21.9 - Prob. 23PCh. 21.9 - Prob. 24PCh. 21.10 - Prob. 25PCh. 21.10 - Prob. 26PCh. 21.SE - Name the following compounds:Ch. 21.SE - Prob. 28VCCh. 21.SE - Prob. 29VCCh. 21.SE - Prob. 30VCCh. 21.SE - Predict the product(s) and provide the mechanism...Ch. 21.SE - Predict the product(s) and provide the mechanism...Ch. 21.SE - Predict the product(s) and provide the mechanism...Ch. 21.SE - Predict the product(s) and provide the complete...Ch. 21.SE - Prob. 35MPCh. 21.SE - When 4-dimethylaminopyridine (DMAP) is added in...Ch. 21.SE - Prob. 37MPCh. 21.SE - Prob. 38MPCh. 21.SE - Prob. 39MPCh. 21.SE - The hydrolysis of a biological thioester to the...Ch. 21.SE - Prob. 41MPCh. 21.SE - Prob. 42MPCh. 21.SE - Prob. 43MPCh. 21.SE - In the iodoform reaction, a triiodomethyl ketone...Ch. 21.SE - Give IUPAC names for the following compounds:Ch. 21.SE - Prob. 46APCh. 21.SE - Draw and name compounds that meet the following...Ch. 21.SE - Predict the product, if any, of reaction between...Ch. 21.SE - Prob. 49APCh. 21.SE - Prob. 50APCh. 21.SE - What product would you expect to obtain from...Ch. 21.SE - Prob. 52APCh. 21.SE - Prob. 53APCh. 21.SE - The following reactivity order has been found for...Ch. 21.SE - Prob. 55APCh. 21.SE - Outline methods for the preparation of...Ch. 21.SE - Prob. 57APCh. 21.SE - When ethyl benzoate is heated in methanol...Ch. 21.SE - tert-Butoxycarbonyl azide, a reagent used in...Ch. 21.SE - Prob. 60APCh. 21.SE - Prob. 61APCh. 21.SE - What is the structure of the polymer produced by...Ch. 21.SE - Polyimides with the structure shown are used as...Ch. 21.SE - Prob. 64APCh. 21.SE - Propose a structure for a compound, C4H7ClO2, that...Ch. 21.SE - Assign structures to compounds with the following...Ch. 21.SE - Prob. 67APCh. 21.SE - When a carboxylic acid is dissolved in...Ch. 21.SE - Prob. 69APCh. 21.SE - Prob. 70APCh. 21.SE - Prob. 71APCh. 21.SE - Phenyl 4-aminosalicylate is a drug used in the...Ch. 21.SE - N,N-Diethyl-m-toluamide (DEET) is the active...Ch. 21.SE - Tranexamic acid, a drug useful against blood...Ch. 21.SE - One frequently used method for preparing methyl...Ch. 21.SE - Prob. 76APCh. 21.SE - Assign structures to compounds with the following...Ch. 21.SE - Propose structures for compounds with the...Ch. 21.SE - Propose a structure for the compound with the...Ch. 21.SE - Draw the structure of the compound that produced...Ch. 21.SE - Prob. 81APCh. 21.SE - Epoxy adhesives are prepared in two steps. SN2...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Given the major organic product(s) of each of the following reactions. If none is predicted, write "N.R." answer] a. CHỊCH, CHẤT AIC13 H b. 0 Cl₂ HC- NHOCH3 FeCl3arrow_forwardGive the major organic product(s) for each of the following reactions or sequences of reactions. Show all relevant stereochemistry [2 ONLY]. A -CH2COOH 1. LIAIH THF, heat 2 HO B. C. CH₂Br Br 1. NaCN, acetone 2 H₂O, heat 1. Mg ether 3 HO Z CO₂arrow_forwardWhat is the order of increasing acidity for the following compounds? (least to most) [2 ONLY] A. COOH COOH COOH COOH 6666 a. IV, I, III, II b. III, II, I, IV с. II, III, I, IV d. III, II, IV, I slingoros CH3 IV woled noise bizarrow_forward
- With this, please answer the following questions: 1.) draw the structure of the electrophilic intermediate in this reaction. 2.) what is the role of the AlCl3 in the reaction 3.) write the complete stepwise mechanism for this reaction. Show all electron flow with arrows and include all intermediate structuresarrow_forwardConsider the data below to answer the following questions. Cyanohydrins are important intermediates in the synthesis of a-hydroxycarboxylic acids from ketones and aldehydes. The nitrile functional group can be hydrolyzed by aqueous acid to yield a carboxylic acid. Nitriles can also be hydrolyzed to carboxylic acids using aqueous base. Unfortunately, when a cyanohydrin is treated with aqueous base the original carbonyl compound is isolated. HO H HCEN H-3- HO' NaOH HO cyanohychin a. a nucleophilic substitution b. an electrophilic addition C10 OH CH-COOH A. The reaction of an aldehyde with hydrogen cyanide is an example of + NaCN + H₂O reaction. H- C. an electrophilic substitution d. a nucleophilic addition B. Identify the electrophile in the reaction of benzaldehyde with hydrogen cyanide.arrow_forwardRefer to the data below to answer the following questions: The octapeptide saralasin is a specific antagonist of angiotensin II. A derivative of saralasin is used therapeutically as an antihypertensive. Amino acid analysis of saralasin show the presence of the following amino acids: Ala, Arg, His, Pro, Sar, Tyr, Val, Val A. Sar is the abbreviation for sarcosine, N-methyl aminoethanoic acid. Draw the structure of sarcosine. B. N-Terminal analysis by the Edman method shows saralasin contains sarcosine at the N-terminus. Partial hydrolysis of saralasin with dilute hydrochloric acid yields the following fragments: Tyr-Val-His Sar-Arg-Val His-Pro-Ala Val-Tyr-Val Arg-Val-Tyr What is the structure of saralasin?arrow_forward
- Give the major organic product(s) of each of the following reactions or sequences of reactions. Show all relevant stereochemistry.[4 only] CH3 A. B. HNO H₂Pt H₂SO4 hano NaN 1. LIAH ether Br 4 2 H₂O C. D. E. CH3CH2-CH2CH3 + HCl Br NH₂ CH3 ON CH-CH3 Br HNOZ CUCI 11,504 HC) 1. HNO H SO NH₂ 2 UMarrow_forwardConsider the Grignard reaction below to answer the following questions. A Mgar 1. ether + MyC CH3 2H3O C B a. The electrophile in this reaction is: b. The nucleophile in this reaction is: c. The alcohol product can be classified as a: a. 1° alcohol b. 2° alcohol C. 3° alcohol d. 4° alcohol HO CH3 CHarrow_forwardGive the major organic product(s) for each of the following reactions or sequences of reactions. Show all relevant stereochemistry A. CH₂OH PCC CH2Cl2 HOO B. H KCN HCN of b C. 1. CH,MgBr, ether 2 HO* D. Choose the BEST reagent for carrying out each of the following conversions. CO₂CH3 CO₂CH3 OH CO₂H сон ن نے a. LiAlH4, ether at abinayo iss c b. NaBH4, ethanol C. CrO3, pyridine d. H₂/Pd d notsiolarrow_forward
- Choose the best reagent for carrying out the following reactions from the list below. Place the letter of the reagent(s) in the box over the reaction arrow. Use only one letter per box. OH OH CH CH CH3 CHS CH3 f OH OCH 3 H A. NaH, then CHI B. C. m-ClC6H4COзH D. E. warm H2SO4/H₂O F. G. H₂/Pd H. I. Cl₂, H₂O J. NaOCH3, CH3OH CH3MgBr in ether, then H3O+ Hg(O2CCF3)2, CH3OH PCC, CH2Cl2 LiAlH4 in ether, then H3O+arrow_forwardWhat is the product of the reaction of 2,4-pentanedione with phenylhydrazine?arrow_forwardIn the reaction of naphthalene with CrO3 in acetic acid. Indicate whether a different product is obtained if carried out at 25°C or with heating (A).arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole
IR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=_TmevMf-Zgs;License: Standard YouTube License, CC-BY