
Concept explainers
Predict the product(s) and provide the mechanism for each reaction below.
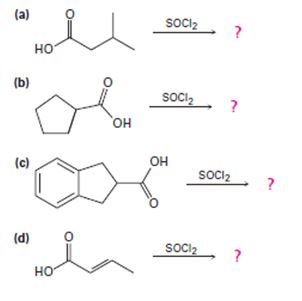
a)
Interpretation:
To predict the mechanism for each reaction of the given products.
Concept introduction:
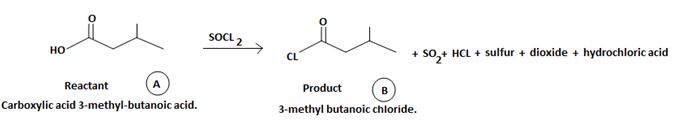
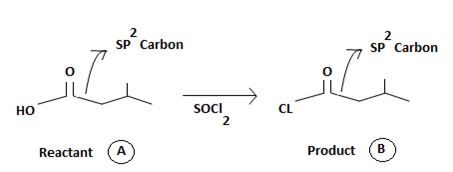
The reaction involves the synthesis of an acid chloride is -3-methyl butanoyl chloride from the carboxylic acid: 3-methylbutanoic acid.
Identify, both, the reactant A and the product B are carboxylic acid derivatives, the common carboxylic acid, an acyl function 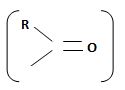 being,
being,
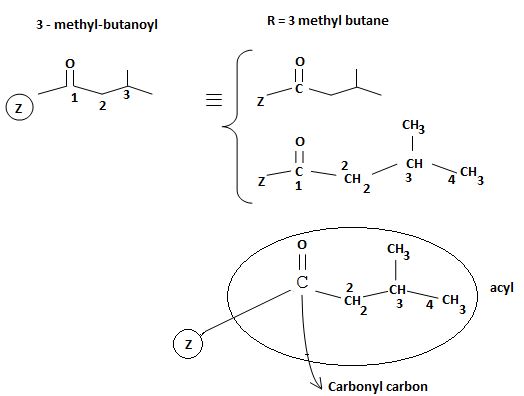
The difference lies in the Z group, ie, the electronegative part. Z is 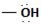 in the reactant A, and
in the reactant A, and 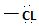 chloride in the product.
chloride in the product.
Both are nucleophiles and overall, the reaction is a transfer of one for the other. Retaining the acyl portion of the molecule.
Answer to Problem 32MP
The product B derives from:
a) Identifying the sp2 carbonyl carbon with the leaving group, is 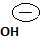 in reactant A
in reactant A
b) Identifying the nucleophile, entering group, is, cl-chloride
c) substitution –cl for the leaving group to get the product B.
There steps, lead to the product.
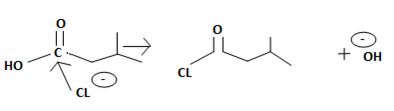
However, mechanistically the reaction, is complex. The reagent given is thionyl chloride.
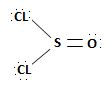
Explanation of Solution
This then must be the source for the attacking Nucleophile ce-, on the carbonyl carbon carrying the leaving group -OH, and of cource, the carbon atom on which the overall nucleophilic acyl substitution occurs. Infact, since acyl chloride are the most reactive of the acid derivatives, they cannot be synthesized from less reactive ones, so special reagents are needed.
In other words, it is not possible, to synthesize the acyl chloride from the acid. Which the given reaction propose. This implies ce- is the weakest base and hence better leaving group than OH. Hence the use of the special reagent thionyl chloride, socl2, an inorganic halide.
socl2 is the acid chloride of the in organic acid sulfurous acid.
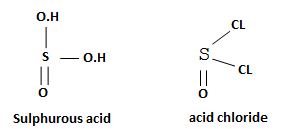
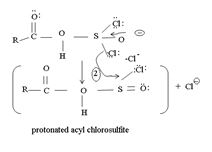
The reaction mechanism involves elimination – Addition by a chloride ion on a highly reactive intermediate; a protonated acyl chlorosulfite. This intermediate contains even better acyl leaving groups than the acyl chloride product. Thionyl chloride reacts in the following way with the acid;-
[gentral: sharing R for the alkyl group]
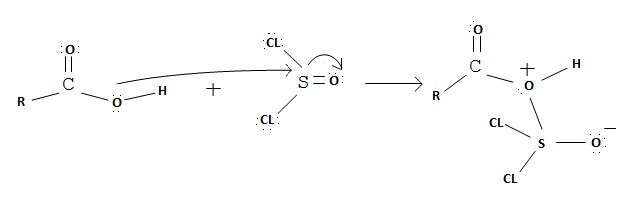
Nucleophilic attack by the oxygen nucleophile on hydroxyl group -OH on sulphur S of thionyl chloride. [S is in the +4 oxidation state and electrondeficient, acide]
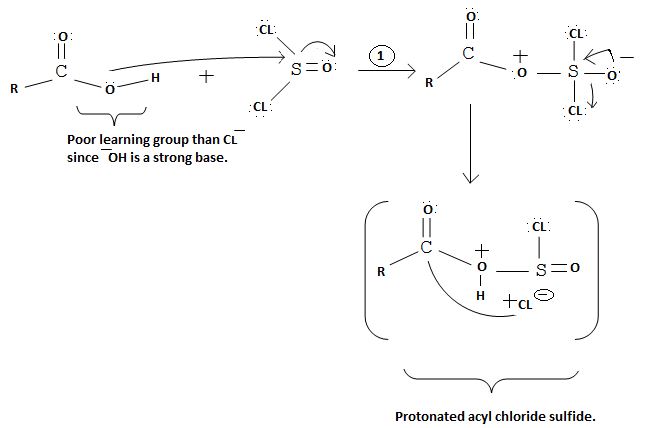
Evidently, the aim of this reaction is to convert the poor leaving group -OH, into a for better leaving group than the final acylchloride product, which it does, as followes:
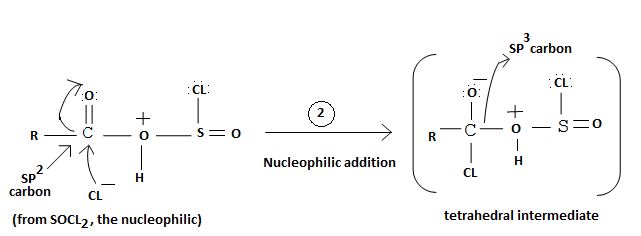
Applying this generalized scheme to the given carboxylic acid is 3-methylbutanoic acid;
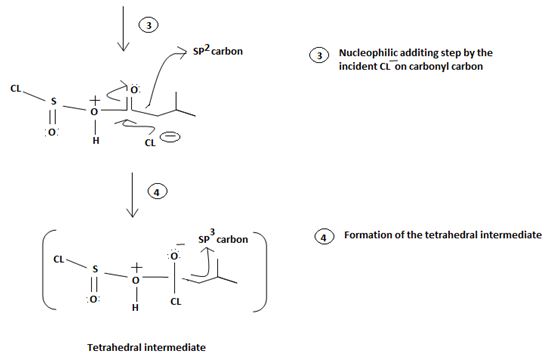
1. Nucleophilic attack of highly electrondeficient S(iv) of socl2
2. Lop of cl generates the potential nucleophilic cl-
3. Nucleophilic adding step by the incipient cl- on carbonyl carbon
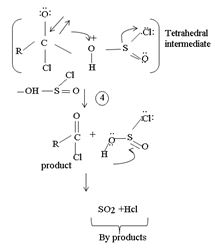
4. Formation of the tetrahedral intermediate tetrahedral intermediate
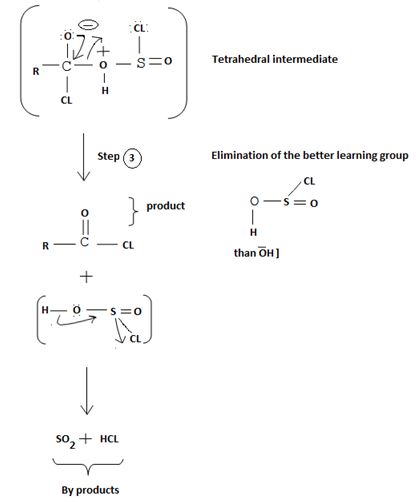
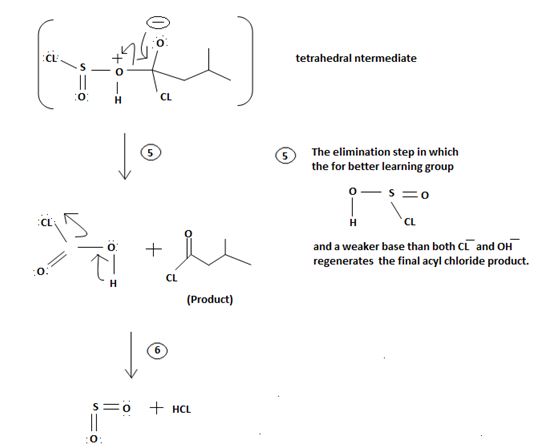
5. The Elimination step in which the for better leaving group  and a weaker base than both cl- and -OH regenerates the final acyl chloride product.
and a weaker base than both cl- and -OH regenerates the final acyl chloride product.
Since SO(OH)cl is unstable, it decomposes into the more stable by-products is SO2 and HCl, all gaseous
Thus the reaction explains the synthesis of the most reactive acyl chloride from a weaker one. It necessarily involves an indirect unusual route using an inorganic acid chloride:
The feasibility arises not only from the use of available starting materials, but also from the fact that the by-products, is Sulphur dioxide and hydrogen chloride are both gaseous.
b)
Interpretation:
To predict the mechanism for each reaction of the given products.
Concept introduction:
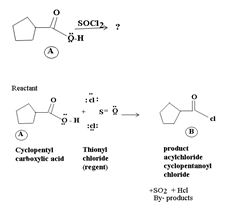
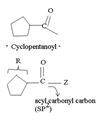
1) The reaction involves the synthesis of an acid-chloride is: cyclopentanol chloride from the carboxylic acid: cyclopentyl carboxylic acid Evidently, both the reactant A and the product B are carboxylic acid derivatives, the common carboxylic acid or acyl function 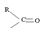 being
being
The difference lies in the Z group, ie. the electronegative part. Z is  in the reactant A, and
in the reactant A, and  ; in the product (chloride). Both are nucleophiles, and overall, the reaction is a replacement of one by the other., retaining the acyl portion of the molecule.
; in the product (chloride). Both are nucleophiles, and overall, the reaction is a replacement of one by the other., retaining the acyl portion of the molecule.
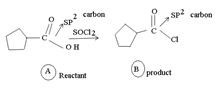
Reaction essentially involves a nucleophilic displacement, but, on an acyl (sp2) carbon atom And, more important, it requires, a weaker bore and hence a better leaving group, on the reactant, and a strong base, a strong entering group a, stronger nucleophile on the product.
However, the given reaction features the reverse; the product halide, in the strongest acyl halide, and cl- is a better leaving group and weakest base than -OH
That the reaction actually occurs, is merely due to unusual reagents employed; is the use of thionyl chloride socl2 an acid halide of the inorganic acid-sulphurous acid.
Answer to Problem 32MP
Before introspecting the, mechanistic details, we derive the product B as follows:
a) Identify the sp2 carbonyl carbon with the leaving group is -OH in reactant,
b) identify the nucleophile cl- (entering group)
c) substitute cl for the leaving group at the acyl carbon to get the product.
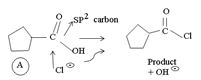
The mechanism, involves Nucleophilic addition followed by elimination using thionyl chloride, is detailed below:
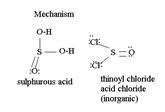
Explanation of Solution
The reaction mechanism involves Elimination-Addition, by a chloride ion on a highly reactive intermediate-a protonated acyl chlorosulphite. This intermediate contains even better acyl leaving groups than the acyl chloride product. Thionyl chloride reacts in the following way with the acid:
[general: showing R for the alkyl group]
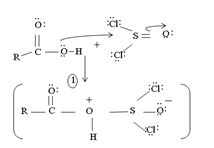
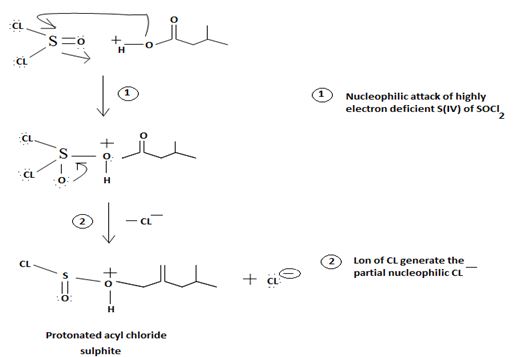
1. Nucleophilic attack by the oxygen nucleophile on the hydroxyl group 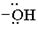 on the Sulphur atom S of thionyl chloride S in socl2 is in the +4 oxidation state and electron deficient, acidic.
on the Sulphur atom S of thionyl chloride S in socl2 is in the +4 oxidation state and electron deficient, acidic.
2. In step 2 loss of chloride ion provides the incipient nucleophile cl-,
Evidently, the aim of this reaction is to convert the poor leaving group 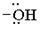 , into a for better leaving group than the final acyl chloride product, which it does, as follows:
, into a for better leaving group than the final acyl chloride product, which it does, as follows:
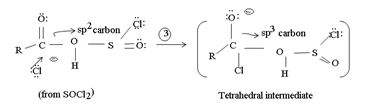
This step is the Nucleophilic addition proper, where the nucleophile cl- from socl2 attack the carbonyl carbon sp2 of the reactant acid to form the tetrahedral intermediate
Applying this generalized scheme to the given carboxylic acid, A thus:
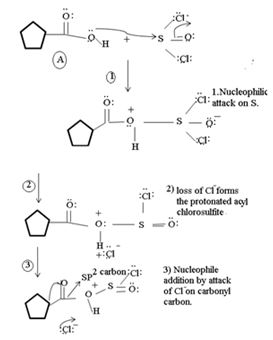
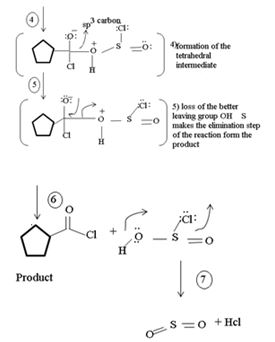
2) Loss of cl- forms the protonated acyl chlorosulfite
3) Nucleophilic addition by attack of cl- on carbonyl carbon formation of the tetrahedral intermediate
4) Elimination of the better leaving group 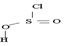 than -OH leads to the product.
than -OH leads to the product.
5) Loss of the better leaving group OH – S = O makes the elimination step of the reaction to form the product
In the final step 7 since 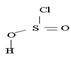 is unstable, it decomposes into the relatives more stable gaseous by-produce so2 and HCl.
is unstable, it decomposes into the relatives more stable gaseous by-produce so2 and HCl.
Thus, the reaction explains the synthesis of the most reactive acyl chloride from a weaker one. It necessarily involves an indirect unusual route using an inorganic acid halide. The feasibility arises not only from the use of readily available starting neutral, but also from the fact that the by-products is so2 and HCl are both gaseous.
c)
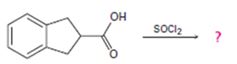
Interpretation:
To predict the mechanism for each reaction of the given products.
Concept introduction:
The reaction involves the synthesis of an acid chloride B from a fused benzene carboxylic acid A. Evidently, both the reactant A and the product B are carboxylic acid derivatives, the common carboxylic acid or acyl function R-C=O being 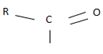 .
.
Answer to Problem 32MP
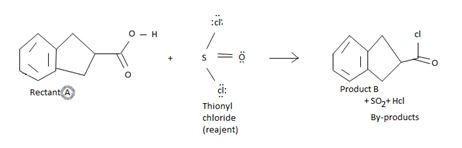
Explanation of Solution
The difference lies in the 2 group: that is the electronegative part. Z is –OH in the reactant A and –cl (chloride) in the product B. Both are nucleophiles, and overall, the reaction is a replacement of one by the other, retaining the acyl portion of the molecules.
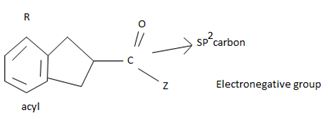
Reaction essentially involves a nucleophilic displacement but, on an acyl (sp2) carbon atom. And more important it requires, a weaker base and hence a better leaving group on the reactant and a strong base a strong entering group on the product.
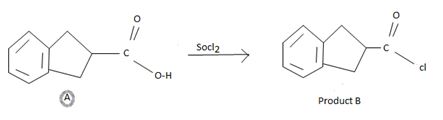
However, the even reaction feature the reverse, the product halide is the strongest acyl halide and cl- is a better leaving group and weakest base than –OH. The fact that the reaction occurs is merely due to unusual reagents being employed by the use of thionyl chloride. Socl2, an acid halide of the inorganic acid –sulphurous acid.
Before, in transporting the mechanistic details, we derive the product B as follows:
Identify the sp2 carboxyl carbon with the leaving group by OH in the reactant.
Identify the nucleophilic cl- (entering group).
Substitute cl for the leaving group at the acyl carbon to get the final product.
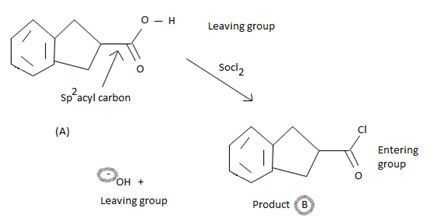
The mechanism involves nucleophilic addition followed by elimination using thionyl chloride is explained below:
The reaction mechanism involves elimination- addition by a chloride ion on a highly reactive inter mediate – a protonated acyl chlorosulphite. This intermediate contains even better acyl leaving groups than final acyl chloride product. Thionylchloride needs as follows:
In step 1
Nucleophilic attack by the oxygen nucleophile on the hydroxyl group –OH of the acid on the highly electron deficient sulphur s(+N) of thionyl chloride.
In step 2
Ion of cl- generate the protonated acyl chloro sulphite a highly reactive reaction intermediate.
Nucleophilic addition now occurs by the incipient cl- from Socl2 on the carboxyl carbon (sp2)of the intermediate. The forms the tetrahedral intermediate of the overall reaction tetrahedral intermediate.
In step 3, the cl- produce from Socl2 is the effective nucleophilic to attack the trigoral sp2 acyl carbon.
Actual tetrahedral intermediate characteristic of nucleophilic acyl substitution.
Step 4 forms the elimination step where the better leaving group departs.
Finally, S(O)(OH)-cl being unstable decomposes to the more stable gaseous products sulphur dioxide and hydrogen chloride Hcl.
Thus the reaction offers a synthetic route for the most reactive acyl halide from the reactive ones by using special inorganic reagents is Socl2. The fleaxibility is further enhanced by the formation of volatile by products.
d)
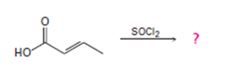
Interpretation:
To predict the mechanism for each reaction of the given products.
Concept introduction:
The reaction involves the synthesis of an acid chloride B from an unsaturated carboxylic acid B. Evidently both the reactant A and the product B are carboxylic acid derivatives, the common carboxylic acid or acyl function 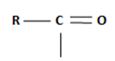 being
being
Answer to Problem 32MP
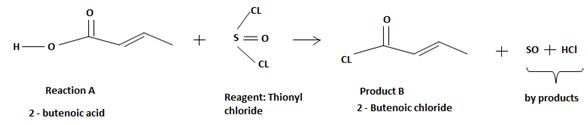
Explanation of Solution
The difference lies in the electronegative part by Z group. Z is –OH (hydroxyl) in the reactant A and chloride –cl in the product B. Both are nucleophiles, and overall. The reaction is a replacement the acyl part of the molecules.
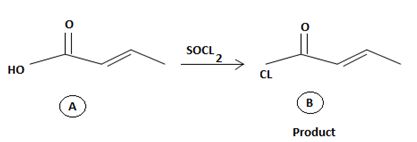
Reaction essentially involves a nucleophilic displacement on an acyl sp2, carbon atom. And more important, it requires a weaker base and hence a better leaving group and the reactant and a strong base a strong entering group on the product.
However the given reaction features the reverse. The product an acyl halide is the strongest carboxylic acid derivative carrying the weakest base and a potentially for too better leaving group than -OH very strong base and very poor leaving group.
The fact then that the reaction occurs is merely due the unusual inorganic reagent being employed by thionyl chloride Socl2. An acid halide of the inorganic sulphurous acid, this reagent effectively transforms the hydroxyl –OH into a very good leaving group and also pupplies the incipient chloride ion cl for nucleophilic attack on the acyl sp2 carbon.
Before intersecting the mechanistic details we derive the product as follows:
a) Identify the sp2 carbonyl carbon atom with the leaving group by –OH in the reactant.
b) Identify the nucleophile cl-(entering group)
c) Substitute cl for the leaving group at the acyl carbon to get the final product
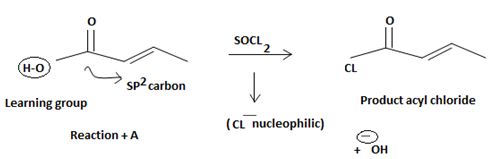
The mechanism involves nucleophilic addition followed by elimination using thionyl chloride is explained below:
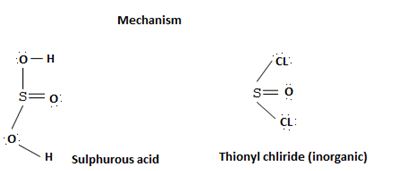
The reaction mechanism involves elimination addition by a chloride ion, cl- on a highly reactive intermediate → a protonated acyl chlorosulphite. This intermediate contains even better acyl leaving groups than the final acyl chloride product. Thionyl chloride reacts as follows:
Step 1
Nucleophilic attack by the oxygen nucleophilic on the hydroxyl group –OH of the acid on the highly electron deficient sulphur (+v) of thionyl chloride.
Step 2
Loss of cl- occurs generating the protonated acyl chlorosulfonate a highly reactive reaction intermediate.
Step 3
The cl- released makes the actual nucleophilic addition step on the acyl carbon sp2 of the acyl group
Tetrahedral inter mediate evidently this forms the tetrahedral intermediate.
Step 4
The elimination step eliminate the good leaving group and forms the product acycle chloride.
Step 5
The unstable by produce further decomposes into the more stable gaseous by produce so2 and Hcl.
Appling this to the general scheme of reactions
1) Nucleophilic attack by OH on s.
2) Loss of cl- forms the reactive acyl chlorosulfonate intermediate.
3) Cl- now attack the acyl carbon tetrahedral intermediate formed.
4) Tetrahedral intermediate.
5) The intermediate soon elimination the leaving group and forms the product.
6) Finally, since it is unstable it decomposes into the more stable gaseous products so2 and Hcl.
Thus, the reaction offers a synthetic route for the most reactive acyl halide from a less reactive one by using spread inorganic reagents eg: Socl2 thionyl chloride.
Evolution of gaseous sulphur dioxide and hydrogen chloride as by products add to the flexibility of the synthetic as no further separation is needed.
Want to see more full solutions like this?
Chapter 21 Solutions
Organic Chemistry
- Draw the line-angle formula of cis-2,3-dichloro-2-pentene. Then, draw the line-angle formula of trans-2,3-dichloro-2-pentene below. Draw the dash-wedge formula of cis-1,3-dimethylcyclohexane. Then, draw the dash-wedge formula of trans-1,3-dimethylcyclohexane below.arrow_forwardRecord the amounts measured and calculate the percent yield for Part 2 in the table below. Dicyclopentadiene measured in volume Cyclopentadiene measured in grams 0 Measured Calculated Mol Yield Mass (g) or Volume (mL) Mass (g) or Volume (ml) 0.6 2.955 Part 2 Measurements and Results Record the amounts measured and calculate the percent yield for Part 2 in the table below. 0.588 0.0044 2.868 0.0434 N/A Table view List view Measured Calculated Mol $ Yield Melting Point (C) Mass (g) or Volume (ml) Mass (g) or Volume (ml.) Cyclopentadiene 0.1 0.08 0.001189 measured in volume Maleic Anhydride 0.196 N/A cis-norbornene-5,6-endo- dicarboxylic anhydride 0.041 0.0002467 N/A N/A N/A 0.002 N/A N/A 128arrow_forwardDraw the condensed structural formula and line-angle formula for each: 2,3-dimethylheptane 3-bromo-2-pentanol 3-isopropyl-2-hexene 4-chlorobutanoic acidarrow_forward
- Record the IUPAC names for each of the structures shown below. a) b) c) OH d) OH e)arrow_forwardA solution of 14 g of a nonvolatile, nonelectrolyte compound in 0.10 kg of benzene boils at 81.7°C. If the BP of pure benzene is 80.2°C and the K, of benzene is 2.53°C/m, calculate the molar mass of the unknown compound. AT₁ = Km (14)arrow_forwardPlease help me answer the following questions. My answers weren't good enough. Need to know whyy the following chemicals were not used in this experiment related to the melting points and kf values. For lab notebook not a graded assignments.arrow_forward
- Draw the arrow pushing reaction mechanism. DO NOT ANSWER IF YOU WONT DRAW IT. Do not use chat gpt.arrow_forwardComplete the following esterification reaction by drawing the structural formula of the product formed. HOH HO i catalyst catalyst OH HO (product has rum flavor) (product has orange flavor)arrow_forwardThe statements in the tables below are about two different chemical equilibria. The symbols have their usual meaning, for example AG stands for the standard Gibbs free energy of reaction and K stands for the equilibrium constant. In each table, there may be one statement that is faise because it contradicts the other three statements. If you find a false statement, check the box next to t Otherwise, check the "no false statements" box under the table. statement false? AG"1 no false statements: statement false? AG-0 0 InK-0 0 K-1 0 AH-TAS no false statements 2arrow_forward
- Complete the following esterification reactions by drawing the line formulas of the carboxylic acid and alcohol required to form the ester shown. catalyst catalyst catalyst apricot fragrancearrow_forwardShow the saponification products of the following ester: You don't need to draw in the Na+ cation. catalyst, A catalyst, A catalyst, Aarrow_forwardWhat would happen if the carboxylic acid and alcohol groups were on the same molecule? In essence, the molecule reacts with itself. Draw the structure of the products formed in this manner using the reactants below. If two functional groups interact with one another on the same molecule, this is called an “intramolecular" (within one) rather than "intermolecular" (between two or more) attack. OH OH catalyst OH HO catalyst catalyst HO OHarrow_forward

 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


