
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 21.4, Problem 11P
Interpretation Introduction
Interpretation:
To Write the reaction between 3,4,5-trimethoxy- benzovl chloride and morpholine to form trimetozine.
Concept introduction:
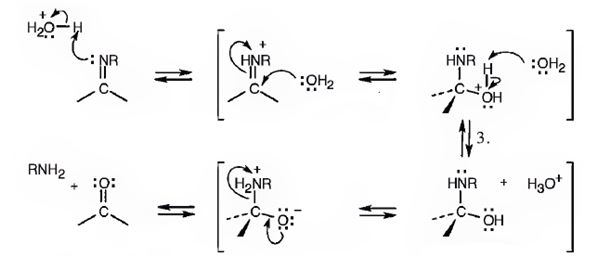
The reaction between 3, 4, 5-trimethoxy- benzoyl chloride and morpholine is shown as
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Name the molecules & Identify any chiral center
CH3CH2CH2CHCH₂CH₂CH₂CH₂
OH
CH₂CHCH2CH3
Br
CH3
CH3CHCH2CHCH2CH3
CH3
Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electrons-pushing arrows for the following reaction or mechanistic step(s).
Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electrons-pushing arrows for the following reaction or mechanistic step(s).
Chapter 21 Solutions
Organic Chemistry
Ch. 21.1 - Give IUPAC names for the following substances:Ch. 21.1 - Draw structures corresponding to the following...Ch. 21.2 - Prob. 3PCh. 21.2 - Rank the compounds in each of the following sets...Ch. 21.2 - Predict the products of the following nucleophilic...Ch. 21.2 - Prob. 6PCh. 21.3 - Prob. 7PCh. 21.3 - If the following molecule is treated with acid...Ch. 21.4 - How might you prepare the following esters using a...Ch. 21.4 - Prob. 10P
Ch. 21.4 - Prob. 11PCh. 21.4 - Prob. 12PCh. 21.4 - Prob. 13PCh. 21.5 - Prob. 14PCh. 21.5 - What product would you expect from reaction of one...Ch. 21.6 - Prob. 16PCh. 21.6 - Prob. 17PCh. 21.6 - Show the products you would obtain by reduction of...Ch. 21.6 - What ester and what Grignard reagent might you...Ch. 21.7 - Prob. 20PCh. 21.7 - How would you use the reaction of an amide with...Ch. 21.8 - Write the mechanism of the reaction shown in...Ch. 21.9 - Prob. 23PCh. 21.9 - Prob. 24PCh. 21.10 - Prob. 25PCh. 21.10 - Prob. 26PCh. 21.SE - Name the following compounds:Ch. 21.SE - Prob. 28VCCh. 21.SE - Prob. 29VCCh. 21.SE - Prob. 30VCCh. 21.SE - Predict the product(s) and provide the mechanism...Ch. 21.SE - Predict the product(s) and provide the mechanism...Ch. 21.SE - Predict the product(s) and provide the mechanism...Ch. 21.SE - Predict the product(s) and provide the complete...Ch. 21.SE - Prob. 35MPCh. 21.SE - When 4-dimethylaminopyridine (DMAP) is added in...Ch. 21.SE - Prob. 37MPCh. 21.SE - Prob. 38MPCh. 21.SE - Prob. 39MPCh. 21.SE - The hydrolysis of a biological thioester to the...Ch. 21.SE - Prob. 41MPCh. 21.SE - Prob. 42MPCh. 21.SE - Prob. 43MPCh. 21.SE - In the iodoform reaction, a triiodomethyl ketone...Ch. 21.SE - Give IUPAC names for the following compounds:Ch. 21.SE - Prob. 46APCh. 21.SE - Draw and name compounds that meet the following...Ch. 21.SE - Predict the product, if any, of reaction between...Ch. 21.SE - Prob. 49APCh. 21.SE - Prob. 50APCh. 21.SE - What product would you expect to obtain from...Ch. 21.SE - Prob. 52APCh. 21.SE - Prob. 53APCh. 21.SE - The following reactivity order has been found for...Ch. 21.SE - Prob. 55APCh. 21.SE - Outline methods for the preparation of...Ch. 21.SE - Prob. 57APCh. 21.SE - When ethyl benzoate is heated in methanol...Ch. 21.SE - tert-Butoxycarbonyl azide, a reagent used in...Ch. 21.SE - Prob. 60APCh. 21.SE - Prob. 61APCh. 21.SE - What is the structure of the polymer produced by...Ch. 21.SE - Polyimides with the structure shown are used as...Ch. 21.SE - Prob. 64APCh. 21.SE - Propose a structure for a compound, C4H7ClO2, that...Ch. 21.SE - Assign structures to compounds with the following...Ch. 21.SE - Prob. 67APCh. 21.SE - When a carboxylic acid is dissolved in...Ch. 21.SE - Prob. 69APCh. 21.SE - Prob. 70APCh. 21.SE - Prob. 71APCh. 21.SE - Phenyl 4-aminosalicylate is a drug used in the...Ch. 21.SE - N,N-Diethyl-m-toluamide (DEET) is the active...Ch. 21.SE - Tranexamic acid, a drug useful against blood...Ch. 21.SE - One frequently used method for preparing methyl...Ch. 21.SE - Prob. 76APCh. 21.SE - Assign structures to compounds with the following...Ch. 21.SE - Propose structures for compounds with the...Ch. 21.SE - Propose a structure for the compound with the...Ch. 21.SE - Draw the structure of the compound that produced...Ch. 21.SE - Prob. 81APCh. 21.SE - Epoxy adhesives are prepared in two steps. SN2...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the IUPAC name of the following compound? CH₂CH₂ H CI H₂CH₂C H CH₂ Selected Answer: O (35,4R)-4 chloro-3-ethylpentane Correctarrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electrons-pushing arrows for the following reaction or mechanistic step(s).arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. I I I H Select to Add Arrows HCI, CH3CH2OHarrow_forward
- Curved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and the follow the arrows to draw the intermediate and product in this reaction or mechanistic step(s).arrow_forwardCurved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the curved arrows to draw the intermediates and product of the following reaction or mechanistic step(s).arrow_forwardCurved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the arrows to draw the intermediate and the product in this reaction or mechanistic step(s).arrow_forward
- Look at the following pairs of structures carefully to identify them as representing a) completely different compounds, b) compounds that are structural isomers of each other, c) compounds that are geometric isomers of each other, d) conformers of the same compound (part of structure rotated around a single bond) or e) the same structure.arrow_forwardGiven 10.0 g of NaOH, what volume of a 0.100 M solution of H2SO4 would be required to exactly react all the NaOH?arrow_forward3.50 g of Li are combined with 3.50 g of N2. What is the maximum mass of Li3N that can be produced? 6 Li + N2 ---> 2 Li3Narrow_forward
- 3.50 g of Li are combined with 3.50 g of N2. What is the maximum mass of Li3N that can be produced? 6 Li + N2 ---> 2 Li3Narrow_forwardConcentration Trial1 Concentration of iodide solution (mA) 255.8 Concentration of thiosulfate solution (mM) 47.0 Concentration of hydrogen peroxide solution (mM) 110.1 Temperature of iodide solution ('C) 25.0 Volume of iodide solution (1) used (mL) 10.0 Volume of thiosulfate solution (5:03) used (mL) Volume of DI water used (mL) Volume of hydrogen peroxide solution (H₂O₂) used (mL) 1.0 2.5 7.5 Time (s) 16.9 Dark blue Observations Initial concentration of iodide in reaction (mA) Initial concentration of thiosulfate in reaction (mA) Initial concentration of hydrogen peroxide in reaction (mA) Initial Rate (mA's)arrow_forwardDraw the condensed or line-angle structure for an alkene with the formula C5H10. Note: Avoid selecting cis-/trans- isomers in this exercise. Draw two additional condensed or line-angle structures for alkenes with the formula C5H10. Record the name of the isomers in Data Table 1. Repeat steps for 2 cyclic isomers of C5H10arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License