
Concept explainers
Predict the product(s) and provide the mechanism for each reaction below.
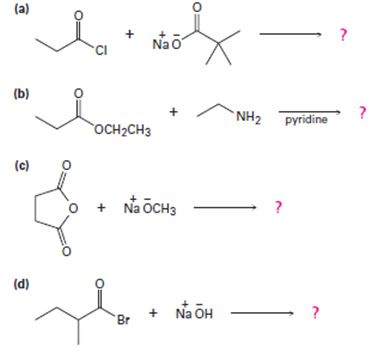
a)
Interpretation:
To predict the mechanism for each reaction of the given products.
Concept introduction:
1) Synthesis of acid derivatives by acyl transfer requires that the reactant has a better learing group at the acyl carbon than the product.
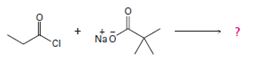
2) The given reaction is a Nucleophilic acyl substitution at the acyl carbon of propanoye chloride 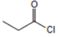 , by Nucleophilic addition elimination mechanism.
, by Nucleophilic addition elimination mechanism.
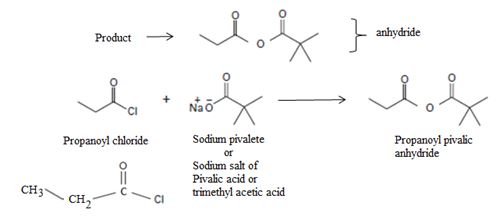
Acyl transfer occurs, overall; ie cl- leaves and is replaced by the new acyl group trimethylacetate 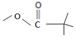 to form the product anhydride.
to form the product anhydride.
Answer to Problem 31MP
Explanation of Solution
1) Reactivity order: [Related to concept 1]
In general, a less reactive acyl group can be synthesized from a more reactive one [but the reverse is usually difficult, and when possible, requires special reajents].
The overall order of reactivity of acyl groups in Nucleophilic acyl substitution is
Thus, acid chlorides are the most reactive in Nu- acyl substitution reaction, and acid amides are the weakest.
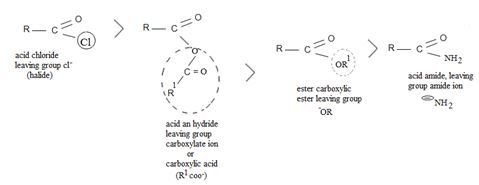
The order is explained on the basis of the relative baricity and hence learning group ability of the bases(g) attached to each acyl carbon 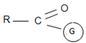 (group).
(group).
Cl- is the weakest base, and so the best learning group. This makes acid chlorides the most reactive. Conversely, the amide ion -NH2 is the strongest base and weakest learning group. Acid amides are thus weakly reacting thus in general, the less reactive acyl compare can be synthesized by Nucleophilic acyl transfer or substitution; which explains the replacement of Cl- by pivalate 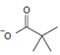 , a weaker group, in the given reaction.
, a weaker group, in the given reaction.
2. Mechanism:
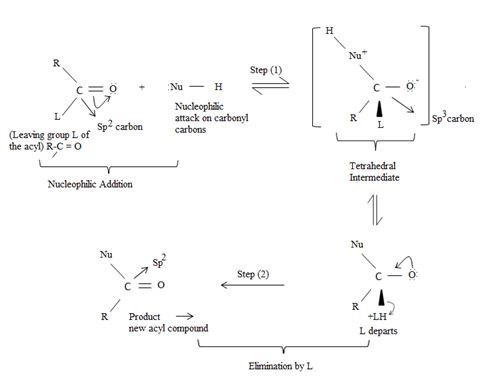
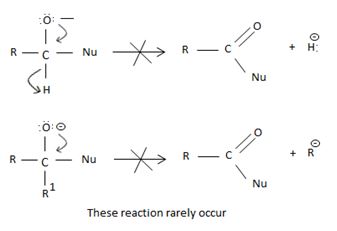
Key to mechanism is the formation of a tetrahedral intermediate [carbon is sp3] that returns to the formes carbonyl group (carbon restored to sp2) after the elimination.
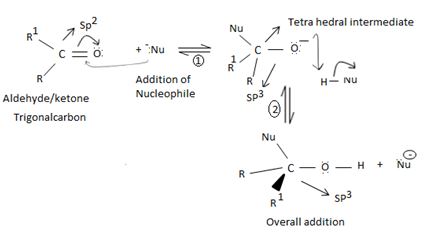
As in Nucleophilic addition to aldehydes/ketones, the initial step in both max reactions involves Nucleophilic addition at the carbonyl carbon.
With both groups of compounds, this initial attack is facilitated by the same factions: the relative static openness of the carbonyl carbon atom and the ability of the carbonyl oxygen atom to accommodate an election pair of the C-O bouble bond.
Following this first step of addition, the two reactions diverge. The tetrahedral intermediate formed from an aldehyde/ketone adopts a proton to form a stable addition product. [carbon remains overall sp3]. In contrast, the intermediate (sp3, tetrahedral) formed from an acyl compound usually eliminates a learning group, potentially good enough, (L) this elimination leads to regeneration of the carbon oxygen double bond and to an overall substitution product.
This difference in behavior (and a major one) stems from the fact that Acyl compounds 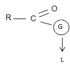 [g=cl-, OCOR, NH2, OR1] all have significantly good potential leaving groups(L) attached to the carbonyl carbon.
[g=cl-, OCOR, NH2, OR1] all have significantly good potential leaving groups(L) attached to the carbonyl carbon.
However, were an aldehyde or ketone to react similarly, by overall elimination, the tetrahedral intermediate would require to eject a hydride ion  , or an alkanide ion
, or an alkanide ion  ; Both are very powerful bases and thus very poor leaving groups.
; Both are very powerful bases and thus very poor leaving groups.
Fitting these mechanistic concepts into the reaction,
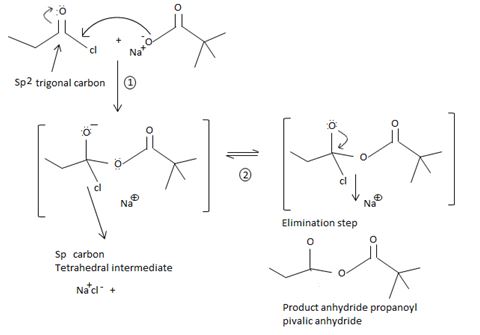
The chloride ion eliminated remains stabilized in the medium by the sodium ion Na+ [large cation, large anion, favourable ionic interaction] as sodium chloride.
Thus in this reaction, the trimethyl carboxylate anion 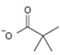 , brings a Nucleophilic acyl substitution at the acyl carbon of propanoyl chloride.
, brings a Nucleophilic acyl substitution at the acyl carbon of propanoyl chloride.
b)
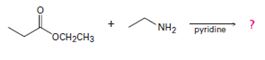
Interpretation:
To predict the mechanism for each reaction of the given products.
Concept introduction:
Product is alkyl substituted amide
1) Synthesis of carboxylic acid derivatives by acyl transfer requires that the reactant has a weaker base and better learning group at its acyl carbon then the product.
In the given reaction, the acyl reactant A has as its leaving group, the ethoxide (-OCH2CH3) (alkoxide) ion, a weaker base than the amide NH2-, the source for nucleophilic attack.
In general, a less reactive acyl group can be synthesized from a more reactive one [the reverse is usually difficult, and may require special reagents when possible].
In the reaction given, the better leaving group weaker base -OCH2CH3 is replaced by the alkylamine CH3 CH2 NH2, the nucleophile.
2) The given reaction is a Nucleophilic acyl substitution at the acyl carbon of the ester 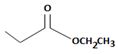 ethyl propanoate.
ethyl propanoate.
Answer to Problem 31MP
Thus, in this reaction the N.alkyl amine 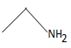 , brings about a Nucleophilic acyl substitution at the acyl carbon of the ester.
, brings about a Nucleophilic acyl substitution at the acyl carbon of the ester.
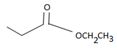 is ethyl propanoate.
is ethyl propanoate.
Explanation of Solution
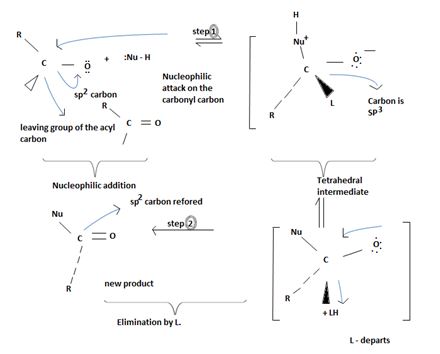
Key to this mechanism is the formation of C tetrahedral intermediate [sp3 Carbon], that returns to the former. [carbon restored to sp2] following the elimination.
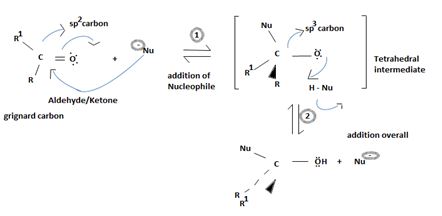
In Nucleophilic addition to aldehydes/ketones, the initial step in both these reactions involves Nucleophilic addition at the carbonyl carbon.
With both groups of compounds, this initial attack is facilitated by the same facting: relative steric openness of the carbonyl carbon atom and the obieuty of the carbonyl carbon atom to accommodate an electron pair of the C-O double bond.
Following this first step of addition, the two reactions diverge. The tetrahedral intermediate formed from an aldehyde/ketone accepts a proton to form a stable addition product. [carbon remains overall sp3]. In contrast, the intermediate (sp3, tetrahedral) formed from an acyl compound usually eliminates a learning group, potentially good, (L)=it is this elimination that leads to regeneration of the carbon oxygen double bond and to an overall substitution product.
This significant difference stems from the fact that all acyl compounds  [g=Cl-, OCOR, NH2, OR1] all have significantly good learning groups L attached to the carbonyl carbon.
[g=Cl-, OCOR, NH2, OR1] all have significantly good learning groups L attached to the carbonyl carbon.
However, were an aldehyde or ketone to react similarly, by overall elimination, the tetrahedral intermediate would require to eliminate C hydride ion H- or an alkanide ion R-; Both are very powerful bases and thus very poor learning groups.
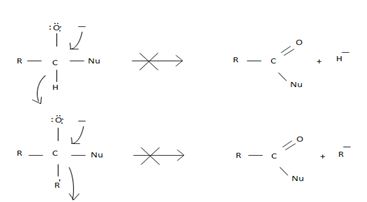
Such reactions rarely occur.
Accomodating these mechanistic concepts into the given reaction
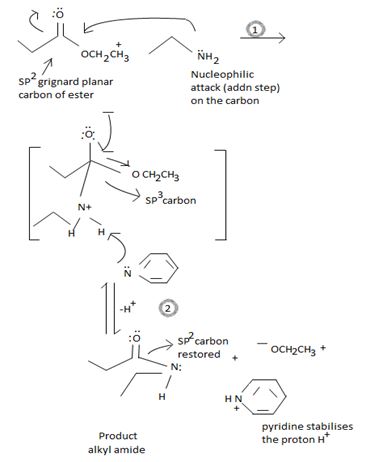
Following the addition step 1, the tetrahedral intermediate in step 2 eliminates the ethoxide ion -OCH2CH3 and the acyl carbon restores to the sp2 state. Meanwhile, the base pyridine present in the medium abstracts a proton of the alkylamine and stabilizes it.
The proton could of course add onto the ethoxide -OCH2CH3, but pyridine being a stronger base abstracts it and acts as an added drawing force.
Moreover addition of a proton [if it all], to -OCH2CH3 would change it to the alcohol, HOCH2CH3, -a poor learning group than the alkoxide, a destalilzing factor in the reaction [though less important] due to the presence of pyridine.
Thus, in this reaction the N.alkyl amine 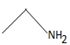 , brings about a Nucleophilic acyl substitution at the acyl carbon of the ester.
, brings about a Nucleophilic acyl substitution at the acyl carbon of the ester.
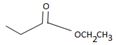 is ethyl propanoate.
is ethyl propanoate.
c)
Interpretation:
To predict the mechanism for each reaction of the given products.
Concept introduction:
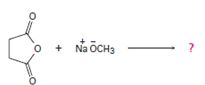
1) Reaction involves Nucleophile acyl substitution, at the acyl carbon, this time of a cyclic anhydride is succinic anhydride.
2) Despite the presence of two carbonyl groups in the cyclicanhydride, the mechanistic pathway followed is the same, as for othercarboxylic acid derivatives. The exception is that: Nucleophile attack occurs at one carbonyl group, while the second carbonyl function becomes part of the leaving group.
General reaction for the Anhydride:
A significant aspect of any cyclic anhydride is that since both “halves” of the anhydride are attached to each other by carbon-carbon bond(s), the acyl compound and the carboxylic acid (by-product) will have to be part of the same molecule. Contrast this in the given reaction, for succinic anhydride.
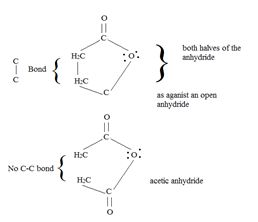
Answer to Problem 31MP
Thus the methoxide ion -OCH3, brings about Nucleophile acyl substitution at an acyl carbon of a carboxylic acid derivative, in this case a cyclic anhydride the synthetic use arises because, as a cyclic anhydride, succinic anhydride can be used to make compounds containing both the acyl group and the carboxyl group: compounds that are for example, both acids and amides, or acids and esters, etc.
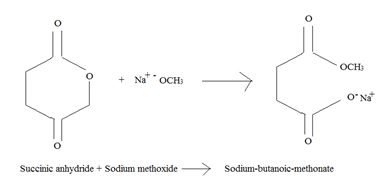
Explanation of Solution
Keeping in view these two concepts, the mechanism for Nucleophile acyl substitution is shown below; first in general and then specifically for succinic anhydride.
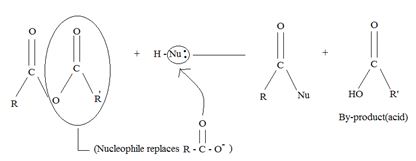
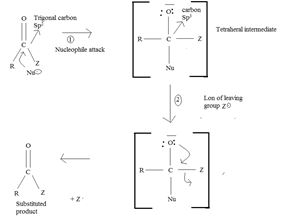
a) General mechanism - Nucleophile acyl substitution
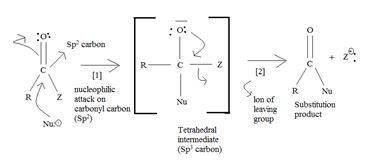
→ In step 1, the Nucleophile Nu, attacks the carbonyl carbon atom (sp2), clearing the C=O bond and forms a tetrahedral intermediate with C new C-Nu bond. This C=O bond cleavage is facilitated (highly favoured) by the relatively more electron equative carbonyl atom; thus the overall electron deficiency of the carbonyl carbon in the acyl function. The potential Nu-: thus attacks this polarized carbon.
In step 2, elimination of the leaving group forms the substitution product.
Thus the overall result of addition of a Nucleophile and elimination of a leaving group is the substitution of a Nucleophile for the leaving group.
Applying for succinic anhydride; the mechanism is:
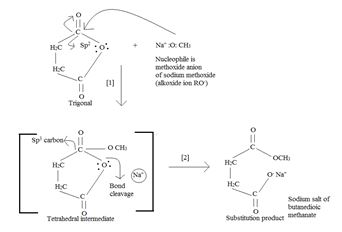
As seen, in step 1, The Nucleophile part, was methoxide ion attcks the positively polarized (sp2) carbon of the acyl function 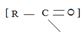 . This forms the tetrahedral intermediate. [Addition step]
. This forms the tetrahedral intermediate. [Addition step]
In step 2, the elimination step, the second half of the anhydride leaves at the C-O Anhydride linkage shown in the intermediate. The acyl carbon is restored to the sp2 state.
The overall product is the both an acid and an ester as shown below. [This time the sodium salt of the acid part]
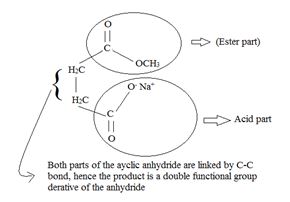
Thus the methoxide ion -OCH3, brings about Nucleophile acyl substitution at an acyl carbon of a carboxylic acid derivative, in this case a cyclic anhydride the synthetic use arises because, as a cyclic anhydride, succinic anhydride can be used to make compounds containing both the acyl group and the carboxyl group: compounds that are for example, both acids and amides, or acids and esters, etc.
d)
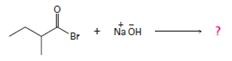
Interpretation:
To predict the mechanism for each reaction of the given products.
Concept introduction:
1. Reaction involves nucleophile acyl substitution at the acyl carbon of 2-methyl-butanoyl bromide, an acyl bromide. The nucleophile is -OH is hydroxide ion, an oxygen nucleophile and also a powerful base.
General Mechanism - Nucleophile acyl substitution
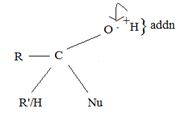
(i) In step 1, the nucleophile: -Nu attacks the trigonal flat, sp2 hydridized of carbonyl carbon of the acyl function 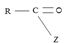 .
.
This carbon is polarized; positive charge, due to the high electron equality of the carbonyl oxygen, is fact it is this partial displacement of the carbonyl π.
Electron cloud, onto oxygen that forms the driving force for the polarization of the C-O π bond; Thus rendering the carbonyl carbon electron deficient and vulnerable to attack by potential nucleophile.
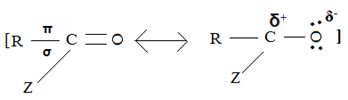
Step 1 is thus the Nucleophile addition step.
(ii) In step 2, elimination of the learning group forms the substitution product.
The overall result of addition of a Nucleophile and elimination of a learning group is substitution of the nucleophile Nu for the learning group Z.
(iii) Further, while in acyl function 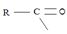 of the reactant
of the reactant 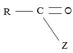 ie carboxylic acid derivative, carbonyl carbon is sp2, trigonal, hybridized that, and sterically open to appropriate potential nucleophile(s) z, in the tetrahedral intermediate that forms, following nucleophile addition, is
ie carboxylic acid derivative, carbonyl carbon is sp2, trigonal, hybridized that, and sterically open to appropriate potential nucleophile(s) z, in the tetrahedral intermediate that forms, following nucleophile addition, is
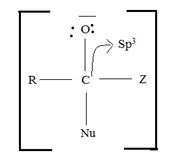
The carbon becomes sp3 hybridized, tetrahedral, and consequently, sterically, just enough saturated.
Answer to Problem 31MP
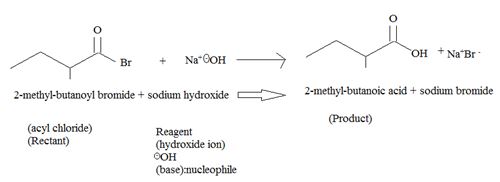
The overall reaction, essentially, a Nucleophile acyl substitution, has led to the synthesis of a less reactive acyl compound, viz : the carboxylic acid 2-methyl-butanoic acid from the more reactive one viz, the acylbromide.
The feasibility of this reaction stems from the potential learning group ability is the Bromide Br-, a weaker base than -OH, and thus a for better leaving group.
Explanation of Solution
Product formation, occurs either by:
1) Loss of z an z-; this depends on the learning group ability of z, in nucleophile acyl-substitution. This elimination regenerates the carbon-oxygen double bond thus leading to a substitutional product.
(or)
2) By the tetrahedral intermediate from an aldehyde or ketone accepts a proton to form a stable addition product.
Applying the above mechanistic concepts, for the given reaction;
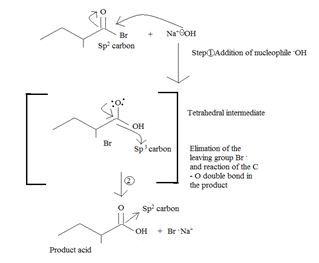
Thus the product formed is 2-methyl butanoic acid.
The weak base Br-, and thus potentially, c good learning group, remains stabilized in solution by the Na+ (ion) [from NaOH] as Na+ Br-, favourable ionic interaction.
The overall reaction, essentially, a Nucleophile acyl substitution, has led to the synthesis of a less reactive acyl compound, viz : the carboxylic acid 2-methyl-butanoic acid from the more reactive one viz, the acylbromide.
The feasibility of this reaction stems from the potential learning group ability is the Bromide Br-, a weaker base than -OH, and thus a for better leaving group.
Want to see more full solutions like this?
Chapter 21 Solutions
Organic Chemistry
- A mixture of C7H12O2, C9H9OCl, biphenyl and acetone was put together in a gas chromatography tube. Please decide from the GC resutls which correspond to the peak for C7,C9 and biphenyl and explain the reasoning based on GC results. Eliminate unnecessary peaks from Gas Chromatography results.arrow_forwardIs the molecule chiral, meso, or achiral? CI .CH3 H₂C CIarrow_forwardPLEASE HELP ! URGENT!arrow_forward
- Identify priority of the substituents: CH3arrow_forwardHow many chiral carbons are in the molecule? OH F CI Brarrow_forwardA mixture of three compounds Phen-A, Acet-B and Rin-C was analyzed using TLC with 1:9 ethanol: hexane as the mobile phase. The TLC plate showed three spots of R, 0.1 and 0.2 and 0.3. Which of the three compounds (Phen-A; Acet-B or Rin-C) would have the highest (Blank 1), middle (Blank 2) and lowest (Blank 3) spot respectively? 0 CH: 0 CH, 0 H.C OH H.CN OH Acet-B Rin-C phen-A A A <arrow_forward

 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


