
Concept explainers
Draw all the structural isomers for C8H18 that have the following root name (longest carbon chain). Name the structural isomers.
a. hexane
b. pentane
(a)
Interpretation: The structural isomers of
Concept introduction: Rules given by IUPAC should be followed to name an organic compound. Any organic compound has only one name that denotes that compound. The root word determines the number of carbons while counting the longest carbon chain. If more than one substituent is present, prefixes like di, tri, tetra, etc. are used and different substituents are written in alphabetical order.
Answer to Problem 16E
Answer
The structural isomers of
Explanation of Solution
Explanation
To determine: The structural isomers of
The structural isomer is given below and its name is
The structure of the isomer is,
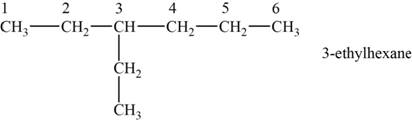
Figure 1
The general formula of alkanes is
Octane has eight carbons and
The isomer has six carbons in the parent chain. Therefore the root word “hexane” is used. Ethyl group is attached to third carbon, thus the name of the isomer is
The structural isomer is given below and its name is
The structure of the isomer is,
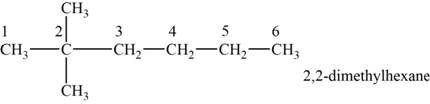
Figure 2
The general formula of alkanes is
Octane has eight carbons and
The isomer has six carbons in the parent chain. Therefore the root word “hexane” is used. Two methyl groups are attached to second carbon, thus the name of the isomer is
The structural isomer is given below and its name is
The structure of the given isomer is,
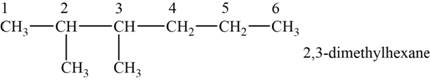
Figure 3
The general formula of alkanes is
Octane has eight carbons and
The isomer has six carbons in the parent chain. Therefore the root word “hexane” is used. Methyl group is attached to second and third carbon, thus the name of the isomer is
The structural isomer is given below and its name is
The structure of the isomer is,
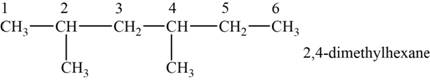
Figure 4
The general formula of alkanes is
Octane has eight carbons and
The isomer has six carbons in the parent chain. Therefore the root word “hexane” is used. Methyl group is attached to second and fourth carbon, thus the name of the isomer is
The structural isomer is given below and its name is
The structure of the isomer is,
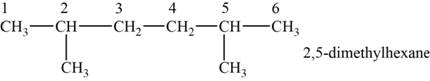
Figure 5
The general formula of alkanes is
Octane has eight carbons and
The isomer has six carbons in the parent chain. Therefore the root word “hexane” is used. Methyl group is attached to second and fifth carbon, thus the name of the isomer is
The structural isomer is given below and its name is
The structure of the isomer is,
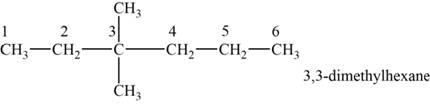
Figure 6
The general formula of alkanes is
Octane has eight carbons and
The isomer has six carbons in the parent chain. Therefore the root word “hexane” is used. Two Methyl groups are attached to third carbon, thus the name of the isomer is
The structural isomer is given below and its name is
The structure of the isomer is,
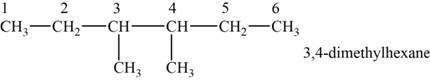
Figure 7
The general formula of alkanes is
Octane has eight carbons and
The isomer has six carbons in the parent chain. Therefore the root word “hexane” is used. Methyl groups are attached to third and fourth carbon, thus the name of the isomer is
Conclusion
The structural isomers of
(b)
Interpretation: The structural isomers of
Concept introduction: Rules given by IUPAC should be followed to name an organic compound. Any organic compound has only one name that denotes that compound. The root word determines the number of carbons while counting the longest carbon chain. If more than one substituent is present, prefixes like di, tri, tetra, etc. are used and different substituents are written in alphabetical order.
Explanation of Solution
Explanation
To determine: The structural isomers of
The structural isomer is given below and its name is
The structure of the isomer is,
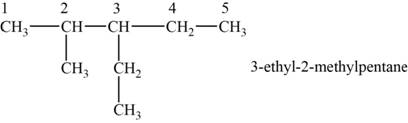
Figure 8
The general formula of alkanes is
Octane has eight carbons and
The isomer has five carbons in the parent chain. Therefore the root word “pentane” is used. Methyl group is attached to second carbon, ethyl group is attached to third carbon, therefore, the name of the isomer is
The structural isomer is given below and its name is
The structure of the isomer is,
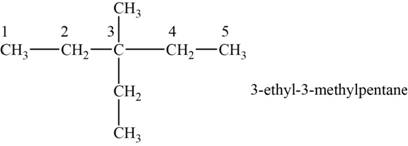
Figure 9
The general formula of alkanes is
Octane has eight carbons and
The isomer has five carbons in the parent chain. Therefore the root word “pentane” is used. Methyl group and ethyl group is attached to third carbon, therefore, the name of the isomer is
The structural isomer is given below and its name is
The structure of the isomer is,
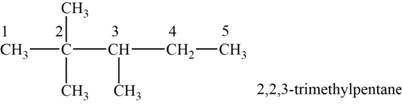
Figure 10
The general formula of alkanes is
Octane has eight carbons and
The isomer has five carbons in the parent chain. Therefore the root word “pentane” is used. Two methyl groups are attached to second carbon and one methyl group is attached to third carbon, therefore, the name of the isomer is
The structural isomer is given below and its name is
The structure of the isomer is,
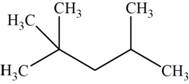
Figure 11
The general formula of alkanes is
Octane has eight carbons and
The isomer has five carbons in the parent chain. Therefore the root word “pentane” is used. Two methyl groups are attached to second carbon and one methyl group is attached to fourth carbon, therefore, the name of the isomer is
The structural isomer is given below and its name is
The structure of the isomer is,
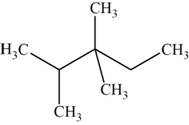
Figure 12
The general formula of alkanes is
Octane has eight carbons and
The isomer has five carbons in the parent chain. Therefore the root word “pentane” is used. Two methyl groups are attached to third carbon and one methyl group is attached to second carbon, therefore, the name of the isomer is
The structural isomer is given below and its name is
The structure of the isomer is,
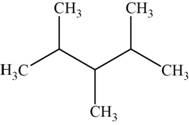
Figure 13
The general formula of alkanes is
Octane has eight carbons and
The isomer has five carbons in the parent chain. Therefore the root word “pentane” is used. Methyl group is attached to second, third carbon and fourth carbon, therefore, the name of the isomer is
Conclusion
The structural isomers of
Want to see more full solutions like this?
Chapter 21 Solutions
EBK CHEMISTRY: AN ATOMS FIRST APPROACH
- Draw a Lewis dot structure for C2H4Oarrow_forward3.3 Consider the variation of molar Gibbs energy with pressure. 3.3.1 Write the mathematical expression for the slope of graph of molar Gibbs energy against 3.3.2 pressure at constant temperature. Draw in same diagram graphs showing variation with pressure of molar Gibbs energies of a substance in gaseous, liquid and solid forms at constant temperature. 3.3.3 Indicate in your graphs melting and boiling points. 3.3.4 Indicate for the respective phases the regions of relative stability.arrow_forwardIn 2-chloropropane, the signal for the H on the C next to Cl should be split into how many peaks?arrow_forward
- 4.4 Consider as perfect gas 3.0 mol of argon gas to which 229 J of energy is supplied as heat at constant pressure and temperature increases by 2.55 K. Calculate 4.4.1 constant pressure molar heat capacity. 4.4.2 constant volume molar heat capacity.arrow_forward3.2 32 Consider calibrating a calorimeter and measuring heat transferred. A sample of compound was burned in a calorimeter and a temperature change of 3.33°C recorded. When a 1.23 A current from a 12.0 V source was passed through a heater in the same calorimeter for 156 s, the temperature changed of 4.47°C was recorded. 3.2.1 Calculate the heat supplied by the heater. 3.2.2 Calculate the calorimeter constant. 3.2.3 Calculate the heat released by the combustion reaction.arrow_forward-.1 Consider the standard enthalpy of formation of gaseous water at 25°C as -241.82 kJ/mol and calculate the standard enthalpy of formation of gaseous water at 100°C.arrow_forward
- 3.5 Complete the following sentences to make correct scientific meaning. 3.5.1 The entropy of a perfect gas. 3.5.2 when it expands isothermally. The change in entropy of a substance accompanying a change of state at its transition 3.5.3 temperature is calculated from its of transition. The increase in entropy when a substance is heated is calculated from itsarrow_forward3.4 Consider the internal energy of a substance 3.4.1 Draw a graph showing the variation of internal energy with temperature at constant volume 3.4.2 Write the mathematical expression for the slope in your graph in 3.4.1arrow_forwardFor a system, the excited state decays to the ground state with a half-life of 15 ns, emitting radiation of 6000 Å. Determine the Einstein coefficients for stimulated absorption and spontaneous emission and the dipole moment of the transition. Data: epsilon 0 = 8.85419x10-12 C2m-1J-1arrow_forward
- Problem a. The following compounds have the same molecular formula as benzene. How many monobrominated products could each form? 1. HC =CC=CCH2CH3 2. CH2=CHC = CCH=CH₂ b. How many dibrominated products could each of the preceding compounds form? (Do not include stereoisomers.)arrow_forwardDon't used Ai solutionarrow_forward4.3 Explain the following terms: 4.3.1 Normal boiling point. 4.3.2 Cooling curve. 4.3.3 Congruent melting. 4.3.4 Ideal solution. 4.3.5 Phase diagram of a pure substance.arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





