
Concept explainers
(a)
Interpretation: By using given bond energies the enthalpy
Concept introduction: When two or more amino acids are linked in a chain, a compound called peptide is formed in which the carboxyl group of each acid is joined to the amino group of the next forming a
To determine: The
(a)
Answer to Problem 130AE
Answer
The
Explanation of Solution
Explanation
The enthalpy
The reaction between two molecules of glycine to form peptide linkage is,
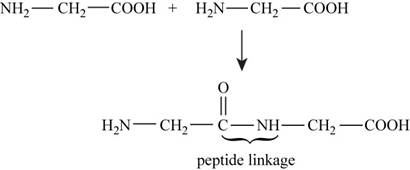
Figure 1
The enthalpy of the reaction is calculated by using the formula,
In the given reaction shown in Figure 1 bonds are broken and formed during peptide formation and energy is absorbed and released. The bond energies are shown in (Table 3-3) respectively.
Therefore, the energy required to break the bonds is calculated as,
Similarly, the energy released when the bonds are formed are calculated as,
Therefore, the total enthalpy for the reaction is calculated by using the formula,
Hence, the total enthalpy of formation for the reaction of two molecules of glycine to form a peptide linkage is positive. It is an endothermic reaction.
(b)
Interpretation: By using given bond energies the enthalpy
Concept introduction: When two or more amino acids are linked in a chain, a compound called peptide is formed in which the carboxyl group of each acid is joined to the amino group of the next forming a
To determine: The entropy
(b)
Answer to Problem 130AE
Answer
The entropy
Explanation of Solution
Explanation
The entropy
The reaction between two molecules of glycine to form a peptide linkage is found to be an endothermic process because of the positive value of enthalpy. In the system an endothermic process absorbs heat from the surrounding and therefore, decreases the entropy of the surrounding because the molecular motion in the surrounding decreases. Therefore, for the given reaction
(c)
Interpretation: By using given bond energies the enthalpy
Concept introduction: When two or more amino acids are linked in a chain, a compound called peptide is formed in which the carboxyl group of each acid is joined to the amino group of the next forming a
To determine: The formation of proteins is a spontaneous process or not.
(c)
Answer to Problem 130AE
Answer
Formation of proteins is a non-spontaneous process.
Explanation of Solution
Explanation
The formation of proteins is a non-spontaneous process.
The formation of proteins occurs when large numbers of amino acids are joined through peptide linkage. The formation of peptide linkage is a non-spontaneous process as stated above due to the negative value of
Want to see more full solutions like this?
Chapter 21 Solutions
EBK CHEMISTRY: AN ATOMS FIRST APPROACH
- What will the enolate for this be using LDA, THF, and cold temperatures? What will it be using NaOEt at rt?arrow_forwardHelp me solve this problem.arrow_forwardDraw a mechanism for the following synthetic transformation including reagents and any isolable intermediates throughout the process. Please clearly indicate bond cleavage/formation using curly arrows. MeO2Carrow_forward
- CHEM 310 Quiz 8 Organic Chemistry II Due: Tuesday, April 25th, at 11:59 pm. This quiz is open textbook / open notes - but you must work alone. You cannot use the internet or the solutions manual for the book. Scan in your work and record an explanation of your mechanism. You may record this any way that you like. One way would be to start an individual Zoom meeting, start recording, "share your screen" and then talk through the problem. This will be converted to an .mp4 file that you can upload into Canvas using the "record/upload media" feature. Pyridine, benzoic acid and benzene are dissolved in ethyl acetate. Design and provide a plan / flow chart for separating and isolating each of these components. Pyridine and benzene are liquids at room temperature. Benzoic acid is a solid. You have ethyl acetate, 2M NaOH, 2M HCI and anhydrous MgSO4 available, as well as all the glassware and equipment that you used in the organic lab this year. Provide accurate acid/base reactions for any…arrow_forwardCan anyone help me solve this step by step. Thank you in advaarrow_forwardPlease draw the mechanism for this Friedel-crafts acylation reaction using arrowsarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning





