
Concept explainers
Interpretation: The structure of 2,3,4,5,6-pentahydroxyhexanal needs to be drawn.
Concept Introduction:
The IUPAC rules are purposed to names different alkanes and alkyl halides.
Answer to Problem 66A
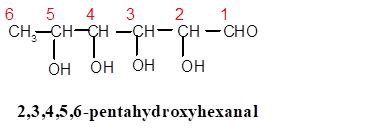
Explanation of Solution
According to IUPAC rule:
- Root word will be determined on the basis of number of C atoms in the longest chain.
- The primary suffix must be determined on the basis of covalent bonds between carbon atoms of the parent chain. They can be −ane, -ene and −yne.
- The secondary suffix indicates the presence of
functional group in the molecule. - The prefix in the IUPAC name indicates the presence of side chain or certain groups like halogens.
In 2,3,4,5,6-pentahydroxyhexanal, the root word is hex thus there must be 6 C atoms in the parent chain with −CHO as main functional group ( secondary suffix is −al) and 5 −OH groups are bonded from C-2 to C-6 therefore the structure must be:
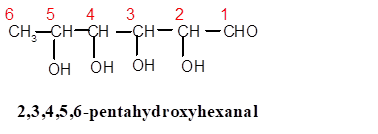
The systematic name is written as follows:
Systematic name = Prefix + Root word + primary suffix + secondary suffix
Chapter 20 Solutions
World of Chemistry
- Pls help asaparrow_forwardPredict the major products of this reaction: ་ ་ + H NaOH ? Δ excess Note that the second reactant is used in excess, that is, there is much more of the second reactant than the first. If there won't be any products, just check the box under the drawing area instead.arrow_forwardP A student claims the right-hand side of the reaction in the drawing area below shows the product of a Claisen condensation. • If the student is correct, complete the reaction by adding the necessary organic reactants to the left-hand side, and by adding any necessary reagents and reaction conditions above and below the arrow. • If the student is incorrect, because it's not possible to obtain this product from a Claisen condensation, check the box under the drawing area instead. those that will minimize any byproducts or competing • Note for advanced students: If you have a choice, use the most efficient reactants and reagents reactions. - ☐ ☐ : ☐ + I Х Click and drag to start drawing a structure.arrow_forward
- identify the relationship between the structures and H- OH HO H H- OH and HO H H -ОН HO H Br and Brarrow_forwardThe right-hand side of this reaction shows the product of an aldol condensation. What are the reactants missing from the left-hand side? Draw them below. ? NaOH Δ If there aren't any reactants that would lead to these products under the reaction conditions given, just check the box under the drawing area. Note for advanced students: don't worry if the reactants you propose might also make some other products under these reaction conditions. Just make sure the product above is one of the major products.arrow_forwardPlease help! I need to identify four labeled unknown bottles based off of their colors doing titration using phenlphtalein. I've included my answers, but I wanted to make sure they were correct and if not, what will be correct thank you in advance.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





