
Concept explainers
(a)
Interpretation:
The structural formula for given alcohol is to be written.
Concept introduction:
The general formula of alcohol is
The alcohols are named by using suffix ‘ol’.
The alcohols are classified as primary, secondary and tertiary on the basis of nature of carbon atom attached to
If carbon containing
If carbon containing
If carbon containing
(a)
Answer to Problem 43A

Explanation of Solution
The prefix ‘ol’ in 1- pentanol shows organic compound contains alcohol
is attached to C-1. The word ‘pentan’ suggest it has five carbon atoms bonded by single bond. So the structural formula is:

1-pentanol is primary alcohol because functional group
(b)
Interpretation:
The structural formula for given alcohol is to be written.
Concept introduction:
The general formula of alcohol is
The alcohols are named by using suffix ‘ol’.
The alcohols are classified as primary, secondary and tertiary on the basis of nature of carbon atom attached to
If carbon containing
If carbon containing
If carbon containing
(b)
Answer to Problem 43A
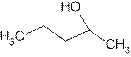
Explanation of Solution
The prefix ‘ol’ in 2- pentanol shows organic compound contains alcohol functional group where
is attached to C-2. The word ‘pentan’ suggest it has five carbon atoms bonded by single bond. So the structural formula is:
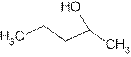
2-pentanol is secondary alcohol because functional group
(c)
Interpretation:
The structural formula for given alcohol is to be written.
Concept introduction:
The general formula of alcohol is
The alcohols are classified as primary, secondary and tertiary on the basis of nature of carbon atom attached to
If carbon containing
If carbon containing
If carbon containing
(c)
Answer to Problem 43A
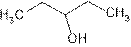
Explanation of Solution
The prefix ‘ol’ in 3- pentanol shows organic compound contains alcohol functional group where
is attached to C-3. The word ‘pentan’ suggest it has five carbon atoms bonded by single bond. So the structural formula is:
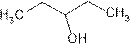
3-pentanol is seconary alcohol because functional group
(d)
Interpretation:
The structural formula for given alcohol is to be written.
Concept introduction:
The general formula of alcohol is
The alcohols are classified as primary, secondary and tertiary on the basis of nature of carbon atom attached to
If carbon containing
If carbon containing
If carbon containing
(d)
Answer to Problem 43A
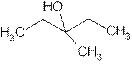
Explanation of Solution
The prefix ‘ol’ in 3-Methyl-3- pentanol shows organic compound contains alcohol functional group where
is attached to C-3. The word ‘pentan’ suggest it has five carbon atoms bonded by single bond. At c-3 one methyl group is attached as a substituent. So the structural formula is:
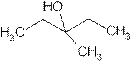
3-Methyl-3- pentanol is tertiary alcohol because functional group
Chapter 20 Solutions
World of Chemistry
- Complete the following acid-base reactions and predict the direction of equilibrium for each. Justify your prediction by citing pK values for the acid and conjugate acid in each equilibrium. (a) (b) NHs (c) O₂N NH NH OH H₁PO₁arrow_forward23.34 Show how to convert each starting material into isobutylamine in good yield. ཅ ནད ཀྱི (b) Br OEt (c) (d) (e) (f) Harrow_forwardPlease help me Please use https://app.molview.com/ to draw this. I tried, but I couldn't figure out how to do it.arrow_forward
- Propose a synthesis of 1-butanamine from the following: (a) a chloroalkane of three carbons (b) a chloroalkane of four carbonsarrow_forwardSelect the stronger base from each pair of compounds. (a) H₂CNH₂ or EtzN (b) CI or NH2 NH2 (c) .Q or EtzN (d) or (e) N or (f) H or Harrow_forward4. Provide a clear arrow-pushing mechanism for each of the following reactions. Do not skip proton transfers, do not combine steps, and make sure your arrows are clear enough to be interpreted without ambiguity. a. 2. 1. LDA 3. H3O+ HOarrow_forward
- b. H3C CH3 H3O+ ✓ H OHarrow_forward2. Provide reagents/conditions to accomplish the following syntheses. More than one step is required in some cases. a. CH3arrow_forwardIdentify and provide an explanation that distinguishes a qualitative and quantitative chemical analysis. Provide examples.arrow_forward
- Identify and provide an explanation of the operational principles behind a Atomic Absorption Spectrometer (AAS). List the steps involved.arrow_forwardInstructions: Complete the questions in the space provided. Show all your work 1. You are trying to determine the rate law expression for a reaction that you are completing at 25°C. You measure the initial reaction rate and the starting concentrations of the reactions for 4 trials. BrO³¯ (aq) + 5Br¯ (aq) + 6H* (aq) → 3Br₂ (l) + 3H2O (l) Initial rate Trial [BrO3] [H*] [Br] (mol/L) (mol/L) | (mol/L) (mol/L.s) 1 0.10 0.10 0.10 8.0 2 0.20 0.10 0.10 16 3 0.10 0.20 0.10 16 4 0.10 0.10 0.20 32 a. Based on the above data what is the rate law expression? b. Solve for the value of k (make sure to include proper units) 2. The proposed reaction mechanism is as follows: i. ii. BrО¸¯ (aq) + H+ (aq) → HBrO3 (aq) HBrO³ (aq) + H* (aq) → H₂BrO3* (aq) iii. H₂BrO³* (aq) + Br¯ (aq) → Br₂O₂ (aq) + H2O (l) [Fast] [Medium] [Slow] iv. Br₂O₂ (aq) + 4H*(aq) + 4Br(aq) → 3Br₂ (l) + H2O (l) [Fast] Evaluate the validity of this proposed reaction. Justify your answer.arrow_forwardе. Д CH3 D*, D20arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





