
Organic Chemistry
12th Edition
ISBN: 9781118875766
Author: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 20, Problem 20P
Give common or systematic names for each of the following compounds:
(a)
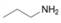
(b)
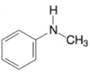
(c)
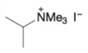
(d)
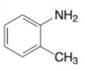
(e)
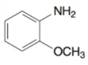
(f)
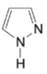
(g)
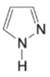
(h)
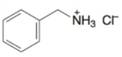
(i)
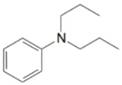
(j)
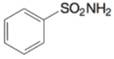
(k)
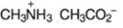
(l)
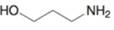
(m)
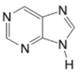
(n)
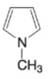
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
5. Draw molecular orbital diagrams for superoxide (O2¯), and peroxide (O2²-). A good starting
point would be MO diagram for O2 given in your textbook. Then: a) calculate bond orders in
superoxide and in peroxide; indicate which species would have a stronger oxygen-oxygen bond;
b) indicate which species would be a radical.
(4 points)
16. Which one of the compunds below is the final product of the reaction sequence
shown here?
عملاء
.OH
Br.
(CH3)2CH-C=C
H+,H,O
2 mol H2, Pt
A
OH
B
OH
D
OH
E
OH
C
OH
Indicate whether any of the two options is correct.a) The most common coordination structure for isopolianions is the prismb) Heteropolianions incorporate alkaline cations into their structures
Chapter 20 Solutions
Organic Chemistry
Ch. 20 - Prob. 1PPCh. 20 - Prob. 2PPCh. 20 - Practice Problem 20.3
Write a mechanism that...Ch. 20 - Prob. 4PPCh. 20 - PRACTICE PROBLEM 20.5 Outline a preparation of...Ch. 20 - Prob. 6PPCh. 20 - Prob. 7PPCh. 20 - Prob. 8PPCh. 20 - Prob. 9PPCh. 20 - Prob. 10PP
Ch. 20 - Practice Problem 20.11 In the preceding examples...Ch. 20 - Prob. 12PPCh. 20 - Prob. 13PPCh. 20 - Practice Problem 20.14
Outline a synthesis of...Ch. 20 - Prob. 15PPCh. 20 - Prob. 16PPCh. 20 - Prob. 17PPCh. 20 - Prob. 18PPCh. 20 - Prob. 19PCh. 20 - 20.20 Give common or systematic names for each of...Ch. 20 - Which is the most basic nitrogen in each compound?...Ch. 20 - Prob. 22PCh. 20 - Prob. 23PCh. 20 - Show how you might synthesize each of the...Ch. 20 - Prob. 25PCh. 20 - 20.26 Provide the major organic product from each...Ch. 20 - Prob. 27PCh. 20 - 20.28 What products would you expect to be formed...Ch. 20 - Prob. 29PCh. 20 - Prob. 30PCh. 20 - Prob. 31PCh. 20 - Write equations for simple chemical rests or state...Ch. 20 - Prob. 33PCh. 20 - Explain the following, including mention of key...Ch. 20 - 20.35 Provide a detailed mechanism for each of the...Ch. 20 - Prob. 36PCh. 20 - Prob. 37PCh. 20 - Prob. 38PCh. 20 - Prob. 39PCh. 20 - 20.40 Give structures for compounds R-W:
Ch. 20 - Prob. 41PCh. 20 - Prob. 42PCh. 20 - Diethylpropion (shown here) is a compound used in...Ch. 20 - Prob. 44PCh. 20 - 20.45 Compound W is soluble in dilute aqueous HCI...Ch. 20 - 20.46 Propose structures for compounds X, Y, and...Ch. 20 - Compound A(C10H15N) is soluble in dilute HCI. The...Ch. 20 - Prob. 48PCh. 20 - Prob. 49PCh. 20 - For each of the following, identify the product...Ch. 20 - 20.51 Develop a synthesis for the following...Ch. 20 - 20.52 When phenyl isochiocyanatc, , is reduced...Ch. 20 - Prob. 53PCh. 20 - 20.54 Propose a mechanism that can explain the...Ch. 20 - When acetone is treated with anhydrous ammonia in...Ch. 20 - Prob. 56P
Additional Science Textbook Solutions
Find more solutions based on key concepts
Why is an endospore called a resting structure? Of what advantage is an endospore to a bacterial cell?
Microbiology: An Introduction
13.2 Describe and give an example (real or hypothetical) of each of the following:
upstream activator sequence...
Genetic Analysis: An Integrated Approach (3rd Edition)
Choose the best answer to each of the following. Explain your reasoning. Tycho Brahes contributions to astronom...
Cosmic Perspective Fundamentals
Interpret the factors which remain constant and variable when a balloon with a volume of 240 mL at 25°C and 1.0...
Living By Chemistry: First Edition Textbook
Why can algae and cyanobacteria be considered indicators of productivity as well as of pollution?
Laboratory Experiments in Microbiology (12th Edition) (What's New in Microbiology)
In Fig. 27-5a, find the potential difference across R2 if = 12 V, R1 = 3.0 , R2 = 4.0 , and R3 = 5.0
Fundamentals of Physics Extended
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please correct answer and don't use hand ratingarrow_forwardWavelength (nm) I'm not sure what equation I can come up with other than the one generated with my graph. Can you please show me the calculations that were used to find this equation? Give an equation that relates energy to wavelength. Explain how you arrived at your equation. Wavelength Energy (kJ/mol) (nm) 350 341.8 420 284.8 470 254.5 530 225.7 580 206.3 620 192.9 700 170.9 750 159.5 Energy vs. Wavelength (Graph 1) 400 350 y=-0.4367x+470.82 300 250 200 150 100 50 O 0 100 200 300 400 500 600 700 800 Energy (kJ/mol)arrow_forward6. For the following molecules: draw Lewis dot-structures; use VSEPR method to determine geometries of the following molecules/ions. Are the central atoms in these molecules/ions considered of normal valency, or are they hypervalent? (please read paragraph 2.6) a) BrF3 (6 points) b) BrF4 c) IF₂ 4arrow_forward
- Nonearrow_forward7. Use Pauling's electronegativity values (Table 1.7) and Ketelaar triangle (Fig. 2.28) to classify bonding in: (3 points) a) CIF3 b) ZnCl2 c) PbSarrow_forward7. What is the IUPAC name of the following compound? A) (R)-1-oxo-2-butanol C) (R)-2-hydroxybutanal E) (S)-1-formyl-1-propanol B) (S)-1-oxo-2-butanol D) (S)-2-hydroxybutanal OH Harrow_forward
- Cual es la formula semidesarrollada del 3-metil-1-butino?arrow_forward2. A graph shown below shows first ionization energies for elements from H to Ne. First ionization energy/kJ mol 2500 2000 1500 1000 500 T T T T 1 2 3 5 6 7 8 9 10 Atomic number a) Using arguments of electronic structure, explain why ionization energy of Li is much lower than that of H. (2 points) then dips at O. b) Using the same arguments, explain why ionization energy increases from B to N, and (3 points)arrow_forwardGive the name of this compound, including stereochemistry if relevant: CICH2 CH3 Br CH₂CH=CH2 Write in the product, including stereochemistry where relevant, for these reactions. See end of ch. 8, p. 301-303. 1. 03 a) 2-methyl-2-pentene -> 2. Zn, H* Br2 b) 1-ethylcyclopentene -->arrow_forward
- Nonearrow_forward3. You may want to read paragraph 1.5 in your textbook before answering this question. Give electron configuration (short-hand notation is fine) for: (5 points) 3+ a) Manganese atom and Mn³+ b) Se atom c) Cu atom and Cu+arrow_forwardPlease correct answer and don't use hand ratingarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

Mass Spectrometry; Author: Professor Dave Explains;https://www.youtube.com/watch?v=hSirWciIvSg;License: Standard YouTube License, CC-BY