
Organic Chemistry
12th Edition
ISBN: 9781118875766
Author: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Question
Chapter 20, Problem 7PP
Interpretation Introduction
Interpretation:
Utilization of the reduction of an amide, oxime, or nitrile to carry out each of the given transformation, is to be shown.
Concept introduction:
▸ Aniline is a primary
The structure of aniline is shown as follows:
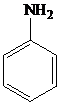
▸ Aniline and substituted aniline reacts with nitrous acid at low temperatures, to form benzene diazonium salts.
▸ The reduction of nitro group results in the formation of primary
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Name
Section
Score
Date
EXERCISE B
pH, pOH, pка, AND PKD CALCULATIONS
1. Complete the following table.
Solution
[H+]
[OH-]
PH
РОН
Nature of Solution
A
2 x 10-8 M
B
1 x 10-7 M
C
D
12.3
6.8
2. The following table contains the names, formulas, ka or pka for some common acids. Fill
in the blanks in the table. (17 Points)
Acid Name
Formula
Dissociation reaction
Ka
pka
Phosphoric acid
H₂PO₁
H3PO4
H++ H₂PO
7.08 x 10-3
Dihydrogen
H₂PO
H₂PO
H+ HPO
6.31 x 10-6
phosphate
Hydrogen
HPO₁
12.4
phosphate
Carbonic acid
H2CO3
Hydrogen
HCO
6.35
10.3
carbonate or
bicarbonate
Acetic acid
CH,COOH
4.76
Lactic acid
CH₂CHOH-
COOH
1.38 x 10
Ammonium
NH
5.63 x 10-10
Phenol
CH₂OH
1 x 10-10
Protonated form
CH3NH3*
3.16 x 10-11
of methylamine
Indicate whether it is true that Co(III) complexes are very stable.
MnO2 acts as an oxidant in the chlorine synthesis reaction.
Chapter 20 Solutions
Organic Chemistry
Ch. 20 - Prob. 1PPCh. 20 - Prob. 2PPCh. 20 - Practice Problem 20.3
Write a mechanism that...Ch. 20 - Prob. 4PPCh. 20 - PRACTICE PROBLEM 20.5 Outline a preparation of...Ch. 20 - Prob. 6PPCh. 20 - Prob. 7PPCh. 20 - Prob. 8PPCh. 20 - Prob. 9PPCh. 20 - Prob. 10PP
Ch. 20 - Practice Problem 20.11 In the preceding examples...Ch. 20 - Prob. 12PPCh. 20 - Prob. 13PPCh. 20 - Practice Problem 20.14
Outline a synthesis of...Ch. 20 - Prob. 15PPCh. 20 - Prob. 16PPCh. 20 - Prob. 17PPCh. 20 - Prob. 18PPCh. 20 - Prob. 19PCh. 20 - 20.20 Give common or systematic names for each of...Ch. 20 - Which is the most basic nitrogen in each compound?...Ch. 20 - Prob. 22PCh. 20 - Prob. 23PCh. 20 - Show how you might synthesize each of the...Ch. 20 - Prob. 25PCh. 20 - 20.26 Provide the major organic product from each...Ch. 20 - Prob. 27PCh. 20 - 20.28 What products would you expect to be formed...Ch. 20 - Prob. 29PCh. 20 - Prob. 30PCh. 20 - Prob. 31PCh. 20 - Write equations for simple chemical rests or state...Ch. 20 - Prob. 33PCh. 20 - Explain the following, including mention of key...Ch. 20 - 20.35 Provide a detailed mechanism for each of the...Ch. 20 - Prob. 36PCh. 20 - Prob. 37PCh. 20 - Prob. 38PCh. 20 - Prob. 39PCh. 20 - 20.40 Give structures for compounds R-W:
Ch. 20 - Prob. 41PCh. 20 - Prob. 42PCh. 20 - Diethylpropion (shown here) is a compound used in...Ch. 20 - Prob. 44PCh. 20 - 20.45 Compound W is soluble in dilute aqueous HCI...Ch. 20 - 20.46 Propose structures for compounds X, Y, and...Ch. 20 - Compound A(C10H15N) is soluble in dilute HCI. The...Ch. 20 - Prob. 48PCh. 20 - Prob. 49PCh. 20 - For each of the following, identify the product...Ch. 20 - 20.51 Develop a synthesis for the following...Ch. 20 - 20.52 When phenyl isochiocyanatc, , is reduced...Ch. 20 - Prob. 53PCh. 20 - 20.54 Propose a mechanism that can explain the...Ch. 20 - When acetone is treated with anhydrous ammonia in...Ch. 20 - Prob. 56P
Knowledge Booster
Similar questions
- In Potassium mu-dihydroxydicobaltate (III) tetraoxalate K4[Co2(C2O4)4(OH)2], indicate whether the OH ligand type is bidentate.arrow_forwardImagine an electrochemical cell based on these two half reactions with electrolyte concentrations as given below: Oxidation: Pb(s) → Pb2+(aq, 0.10 M) + 2 e– Reduction: MnO4–(aq, 1.50 M) + 4 H+(aq, 2.0 M) + 3 e– → MnO2(s) + 2 H2O(l) Calculate Ecell (assuming temperature is standard 25 °C).arrow_forward: ☐ + Draw the Fischer projection of the most common naturally-occurring form of aspartate, with the acid group at the top and the side chain at the bottom. Important: be sure your structure shows the molecule as it would exist at physiological pH. Click and drag to start drawing a structure. ✓arrow_forward
- For a silver-silver chloride electrode, the following potentials are observed: E°cell = 0.222 V and E(saturated KCl) = 0.197 V Use this information to find the [Cl–] (technically it’s the activity of Cl– that’s relevant here, but we’ll just call it “concentration” for simplicity) in saturated KCl.arrow_forwardA concentration cell consists of two Sn/Sn2+ half-cells. The cell has a potential of 0.10 V at 25 °C. What is the ratio of [Sn2+] (i.e., [Sn2+left-half] / [Sn2+right-half])?arrow_forwardElectrochemical cell potentials can be used to determine equilibrium constants that would be otherwise difficult to determine because concentrations are small. What is Κ for the following balanced reaction if E˚ = +0.0218 V? 3 Zn(s) + 2 Cr3+(aq) → 3 Zn2+(aq) + Cr(s) E˚ = +0.0218 Varrow_forward
- Consider the following half-reactions: Hg2+(aq) + 2e– → Hg(l) E°red = +0.854 V Cu2+(aq) + 2e– → Cu(s)E°red = +0.337 V Ni2+(aq) + 2e– → Ni(s) E°red = -0.250 V Fe2+(aq) + 2e– → Fe(s) E°red = -0.440 V Zn2+(aq) + 2e– → Zn(s) E°red = -0.763 V What is the best oxidizing agent shown above (i.e., the substance that is most likely to be reduced)?arrow_forwardCalculate the equilibrium constant, K, for MnO2(s) + 4 H+(aq) + Zn(s) → Mn2+(aq) + 2 H2O(l) + Zn2+(aq)arrow_forwardIn the drawing area below, draw the condensed structures of formic acid and ethyl formate. You can draw the two molecules in any arrangement you like, so long as they don't touch. Click anywhere to draw the first atom of your structure. A C narrow_forward
- Write the complete common (not IUPAC) name of each molecule below. Note: if a molecule is one of a pair of enantiomers, be sure you start its name with D- or L- so we know which enantiomer it is. molecule Ο C=O common name (not the IUPAC name) H ☐ H3N CH₂OH 0- C=O H NH3 CH₂SH H3N ☐ ☐ X Garrow_forward(Part A) Provide structures of the FGI products and missing reagents (dashed box) 1 eq Na* H* H -H B1 B4 R1 H2 (gas) Lindlar's catalyst A1 Br2 MeOH H2 (gas) Lindlar's catalyst MeO. OMe C6H1402 B2 B3 A1 Product carbons' origins Draw a box around product C's that came from A1. Draw a dashed box around product C's that came from B1.arrow_forwardClassify each of the amino acids below. Note for advanced students: none of these amino acids are found in normal proteins. X CH2 H3N-CH-COOH3N-CH-COO- H3N-CH-COO CH2 CH3-C-CH3 CH2 NH3 N NH (Choose one) ▼ (Choose one) S CH2 OH (Choose one) ▼ + H3N-CH-COO¯ CH2 H3N CH COO H3N-CH-COO CH2 오오 CH CH3 CH2 + O C CH3 O= O_ (Choose one) (Choose one) ▼ (Choose one) Garrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning