
Concept explainers
(a)
Interpretation:
The VSEPR formula and shape for sulfur tetrachloride molecule have to be predicted.
Concept Introduction:
Valence Shell Electron Pair Repulsion model predicts shape by inclusion of bond angles and most distant arrangement of atoms that leads to minimum repulsion. For the molecules that have no lone pairs around the central atom the bonded-atom unshared -pair arrangement is decided by the table as follows:
In order to determine the shape the steps to be followed are indicated as follows:
- 1. Lewis structure of molecule should be written.
- 2. The type electron arrangement around the central atom should be identified around the central atom. This essentially refers to determination of bond pairs and unshared or lone pairs around central atoms.
- 3. Then bonded-atom unshared -pair arrangement that can maximize the distance of electron pairs about central atom determines the shape.
For molecules that have lone pairs around central atom, lone pairs influence shape, because there are no atoms at the positions occupied by these lone pairs. The key rule that governs the molecular shape, in this case, is the extent of lone –lone pair repulsions are far greater than lone bond pair or bond pair-bond pair repulsions. The table that summarized the molecular shapes possible for various combinations of bonded and lone pairs are given as follows:
(a)
Answer to Problem 2E.11E
The shape for sulfur tetrachloride molecule is square planar and VSEPR formula is
Explanation of Solution
Sulfur tetrachloride has sulphur as central atom. Sulfur has six valence electrons while chlorine possesses seven valence electrons.
Total valence electrons are sum of the valence electrons on each chlorine and central sulfur in
The skeleton structure in
These 13 electron pairs are assigned as lone pairs of each of the chlorine atoms to satisfy its octet.
Hence, the Lewis structure of
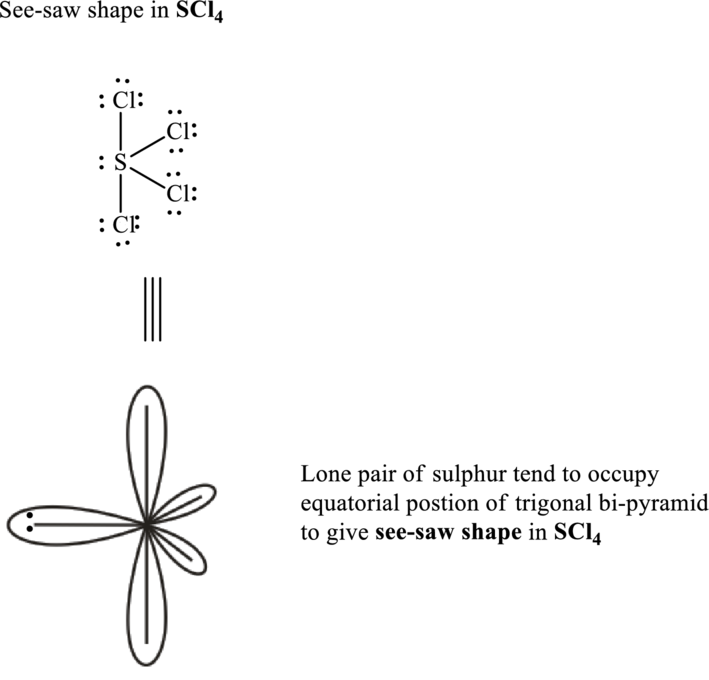
It is evident that in
If lone pairs are represented by E, central atom with A and other attached bond pairs by X, then for any square planar species the VSEPR formula is predicted as
(b)
Interpretation:
The VSEPR formula and shape for iodine trichloride molecule have to be predicted.
Concept Introduction:
Refer to part (a).
(b)
Answer to Problem 2E.11E
The shape for iodine trichloride is T-shape and VSEPR formula is
Explanation of Solution
Iodine trichloride has
Total valence electrons are sum of the valence electrons on each chlorine and central iodine in
The skeleton structure in
These 11 electron pairs are allotted as lone pairs of each of the chlorine atoms to satisfy its octet. Hence, the Lewis structure and corresponding T-shape in
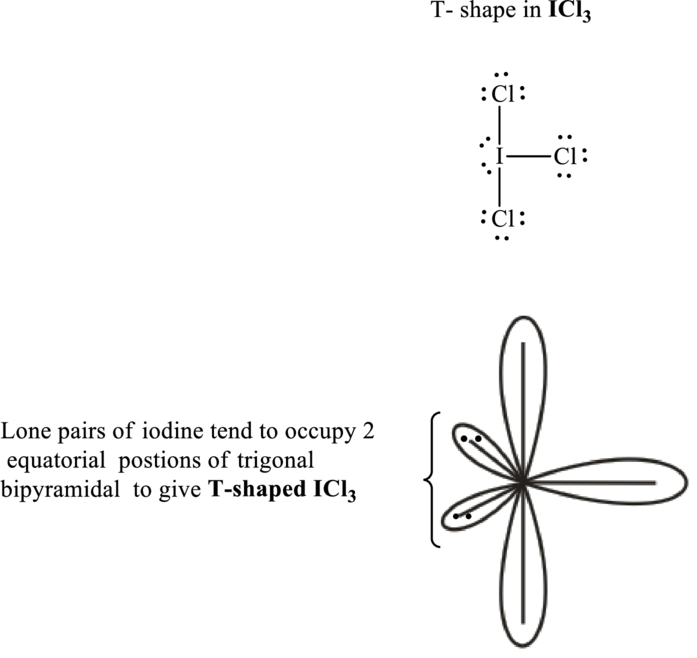
It is evident that in
If lone pairs are represented by E, central atom with A and other attached bond pairs by X, then for any bent or T-shaped species the VSEPR formula is predicted to be
(c)
Interpretation:
The VSEPR formula and shape for
Concept Introduction:
Refer to part (a).
(c)
Answer to Problem 2E.11E
The shape for
Explanation of Solution
Total valence electrons are sum of the valence electrons on each fluorine and central iodine in
The skeleton structure in
These 14 electron pairs are allotted as lone pairs of each of the fluorine atoms to satisfy its octet. Hence, the Lewis structure and corresponding T-shape in
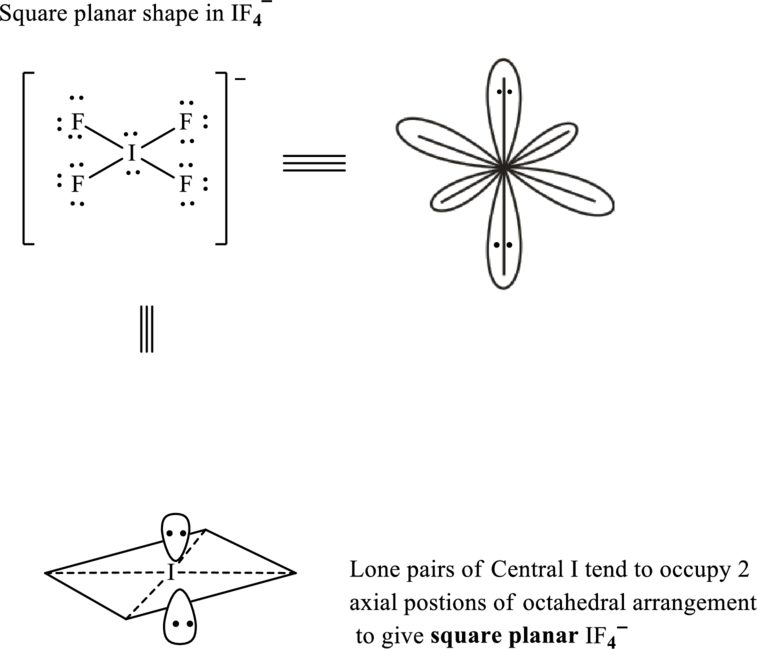
In
If lone pairs are represented by E, central atom with A and other attached bond pairs by X, then for any square planar species the VSEPR formula is predicted as
(d)
Interpretation:
The VSEPR formula and shape for xenon trioxide molecule have to be predicted.
Concept Introduction:
Refer to part (a).
(d)
Answer to Problem 2E.11E
The shape for xenon trioxide molecule is trigonal pyramidal and corresponding VSEPR formula is
Explanation of Solution
Xenon trioxide has
Total valence electrons are sum of the valence electrons on each oxygen atom and central
The skeleton structure in
These 7 electron pairs are allotted as lone pairs of each of the oxygen atoms to satisfy its octet. Thus, the Lewis structure and shape of
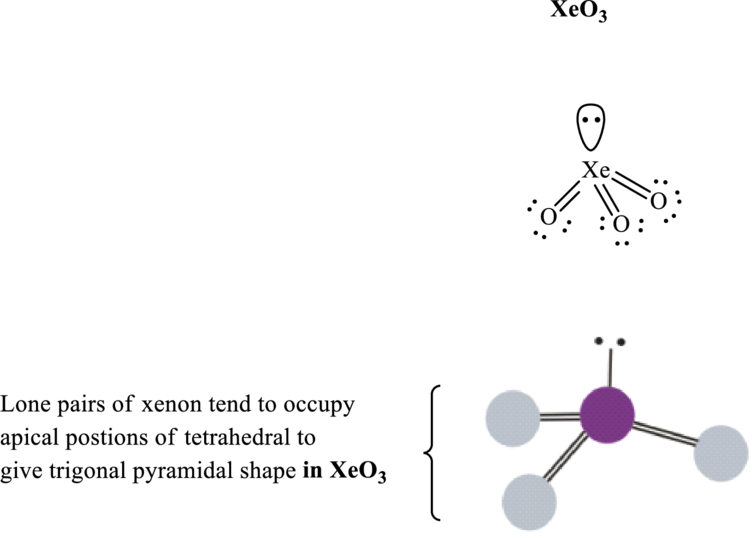
It is evident that in
If lone pairs are represented by E, central atom with A and other attached bond pairs by X, then for trigonal pyramidal any species the VSEPR formula is predicted as
Want to see more full solutions like this?
Chapter 2 Solutions
ACHIEVE/CHEMICAL PRINCIPLES ACCESS 2TERM
- For Raman spectroscopy/imaging, which statement is not true regarding its disadvantages? a) Limited spatial resolution. b) Short integration time. c) A one-dimensional technique. d) Weak signal, only 1 in 108 incident photons is Raman scattered. e) Fluorescence interference.arrow_forwardUsing a cell of known pathlength b = 1.25115 x 10-3 cm, a water absorption spectrum was measured. The band at 1645 cm-1, assigned to the O-H bending, showed an absorbance, A, of 1.40. a) Assuming that water density is 1.00 g/mL, calculate the water molar concentration c (hint: M= mole/L) b) Calculate the molar absorptivity, a, of the 1645 cm-1 band c) The transmitted light, I, can be written as I= Ioexp(-xb), where x is the absorption coefficient (sometimes designated as alpha), Io is the input light, and b is the cell pathlength. Prove that x= (ln10)*x*c. (Please provide a full derivation of the equation for x from the equation for I). d) Calculate x for the 1645 cm-1 bandarrow_forwardI need help with the follloaingarrow_forward
- For a CARS experiment on a Raman band 918 cm-1, if omega1= 1280 nm, calculate the omega2 in wavelength (nm) and the CARS output in wavelength (nm).arrow_forwardI need help with the following questionarrow_forwardFor CARS, which statement is not true regarding its advantages? a) Contrast signal based on vibrational characteristics, no need for fluorescent tagging. b) Stronger signals than spontaneous Raman. c) Suffers from fluorescence interference, because CARS signal is at high frequency. d) Faster, more efficient imaging for real-time analysis. e) Higher resolution than spontaneous Raman microscopy.arrow_forward
- Draw the major product of the Claisen condensation reaction between two molecules of this ester. Ignore inorganic byproducts. Incorrect, 5 attempts remaining 1. NaOCH3/CH3OH 2. Acidic workup Select to Draw O Incorrect, 5 attempts remaining The total number of carbons in the parent chain is incorrect. Review the reaction conditions including starting materials and/or intermediate structures and recount the number of carbon atoms in the parent chain of your structure. OKarrow_forwardUsing a cell of known pathlength b = 1.25115 x 10-3 cm, a water absorption spectrum was measured. The band at 1645 cm-1, assigned to the O-H bending, showed an absorbance, A, of 1.40. a) Assuming that water density is 1.00 g/mL, calculate the water molar concentration c (hint: M= mole/L) b) Calculate the molar absorptivity, a, of the 1645 cm-1 band c) The transmitted light, I, can be written as I= Ioexp(-xb), where x is the absorption coefficient (sometimes designated as alpha), Io is the input light, and b is the cell pathlength. Prove that x= (ln10)*x*c d) Calculate x for the 1645 cm-1 bandarrow_forwardConvert 1.38 eV into wavelength (nm) and wavenumber (cm-1) (c = 2.998 x 108 m/s; h = 6.626 x 10-34 J*s).arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
