
Concept explainers
(a)
Interpretation:
The formal charge on each atom has to be determined and structure of lower energy has to be identified.
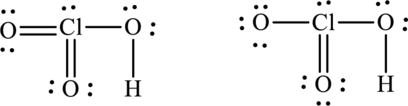
Concept Introduction:
The formal charge on each atom in the Lewis structure can be calculated from the equation written as follows:
Here,
Lowest energy structure is the one that has zero or nearly zero formal charge.
(a)
Explanation of Solution
The formal charge on each atom in the Lewis structure can be calculated from the equation as follows:
For first structure:
Substitute 6 for
Substitute 6 for
Substitute 7for
Substitute 1 for
For first structure:
Similarly for other molecule substitute 6 for
Substitute 6 for
Substitute 7 for
Substitute 1 for
Hence formal charge in the right structure is illustrated below:
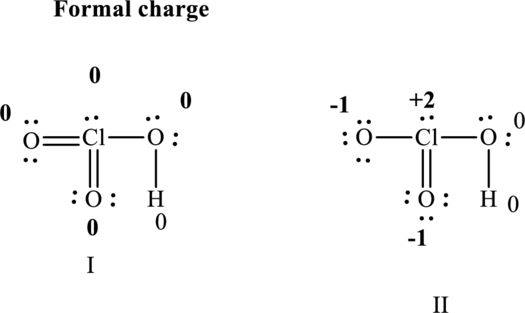
Since the former hasformal charges close to zero for maximum number of atoms I is lower in energy than II.
(b)
Interpretation:
The formal charge on each atom has to be determined and structure of lower energy has to be identified.

Concept Introduction:
Refer to part (a).
(b)
Explanation of Solution
The formal charge on each atom in the Lewis structure can be calculated from the equation as follows:
Substitute 6 for
Substitute 6 for
Substitute 4for
Similarly for other Lewis structure, substitute 6 for
Substitute 6 for
Substitute 4 for
Hence formal charge in the two structures is illustrated below:
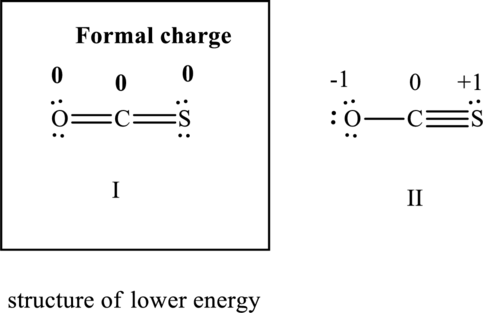
Since the former has all formal charges equal to zero I is lower in energy than II hence I represents structure of lower energy.
(b)
Interpretation:
The formal charge on each atom has to be determined and structure of lower energy has to be identified.

Concept Introduction:
Refer to part (a).
(b)
Explanation of Solution
The formal charge on each atom in the Lewis structure can be calculated from the equation as follows:
Substitute 1 for
Substitute 4 for
Substitute 5for
Similarly for other Lewis structure, substitute 1 for
Substitute 4 for
Substitute 5 for
Hence formal charge in the two structures is illustrated below:
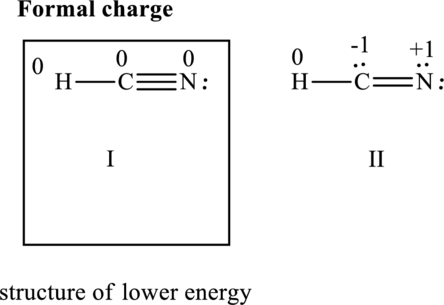
Since the former has all formal charges equal to zero I is lower in energy than II hence I represents structure of lower energy.
Want to see more full solutions like this?
Chapter 2 Solutions
ACHIEVE/CHEMICAL PRINCIPLES ACCESS 2TERM
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning





