
Concept explainers
(a)
Interpretation:
Lewis structure of
Concept Introduction:
Lewis structures represent covalent bonds and describe valence electrons configuration of atoms. The covalent bonds are depicted by lines and unshared electron pairs by pairs of dots. The sequence to write Lewis structure of some molecule is given as follows:
- The central atom is identified and various other atoms are arranged around it. This central atom so chosen is often the least electronegative.
- Total valence electrons are estimated for each atom.
- single bond is first placed between each atom pair.
- The electrons left can be allocated as unshared electron pairs or as multiple bonds around the
symbol of the element to satisfy the octet (or duplet) for each atom. - Add charge on the overall structure in case of polyatomic cation or anion.
(a)
Explanation of Solution
Hence, 6 pairs are to be allocated to form the Lewis structure of
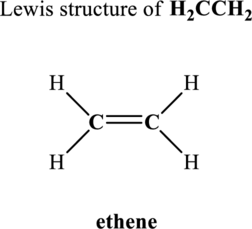
Similarly, total valence electrons is sum of the valence electrons for each atom along with a charge in
Hence, 8 pairs are to be allocated to form the Lewis structure of
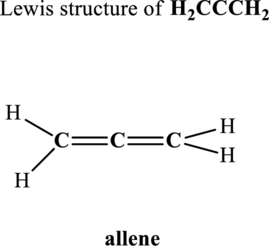
Likewise, total valence electrons in
Hence, 10 pairs are to be allocated to form the Lewis structure of
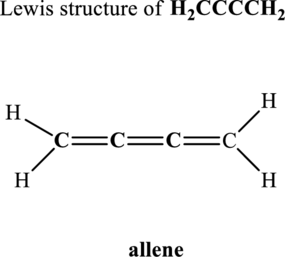
(b)
Interpretation:
The hybridization at each carbon in
Concept Introduction:
The table that relates the steric number with hybridization is as follows:
(b)
Explanation of Solution
In the structure of
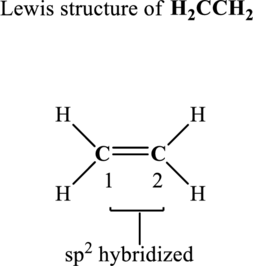
The terminal carbon atoms at positions 1 and 2 are trigonal planar with 3 as steric number hence the hybridization of these carbon atoms is
In the structure of
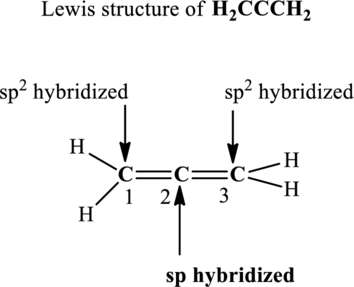
Since the steric number around central carbon is 2 therefore, the hybridization of carbon atom located at positions 2 is
In the structure of
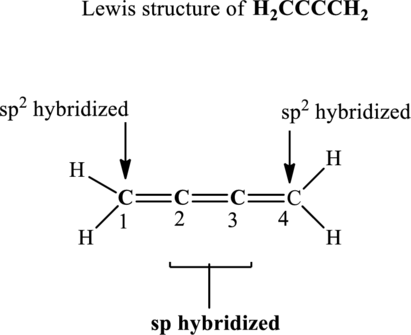
Central carbon atoms have effectively two bond pairs as each double bond is regarded as one single bond pair. Since the steric number around central carbon atoms is 2 therefore, the hybridization of carbon atoms located at positions 2 and 3 is
The terminal carbon atoms at positions 1 and 4 are trigonal planar with 3 as steric number hence the hybridization of this carbon is
(c)
Interpretation:
The type of bonds that connect carbon atoms in
Concept Introduction:
A single bond is always associated with sigma bonds. Molecules that have all atoms connected by sigma bond exclusively are stated to be in
A double bond and carbocation are associated with
A triple bond or central carbon structure similar to allene is always associated with
(c)
Explanation of Solution
In
In
In
(d)
Interpretation:
The bond angles in
Concept Introduction:
Refer to part (c).
(d)
Explanation of Solution
A single bond is always associated with sigma bonds. Molecules that have all atoms connected by sigma bond exclusively are stated to be in
A double bond and carbocation are associated with
A triple bond or central carbon structure similar to allene is always associated with
Thus the bond angles can be illustrated as follows:
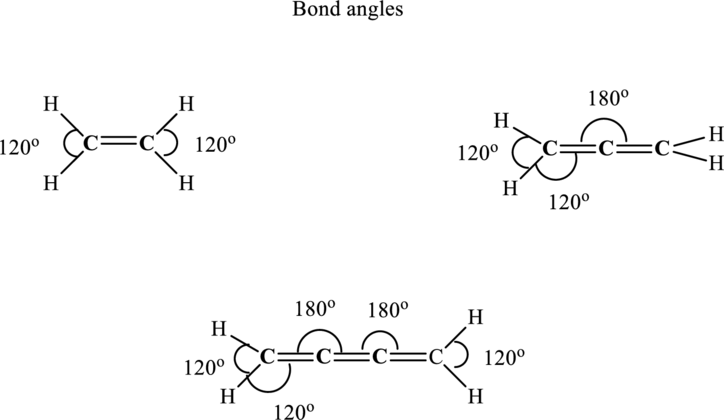
(e)
Interpretation:
Whether all hydrogen atoms lie in the same plane or not in
Concept Introduction:
Refer to part (c).
(e)
Explanation of Solution
In allene, the central carbon that is double bonded to each carbon in different planes is
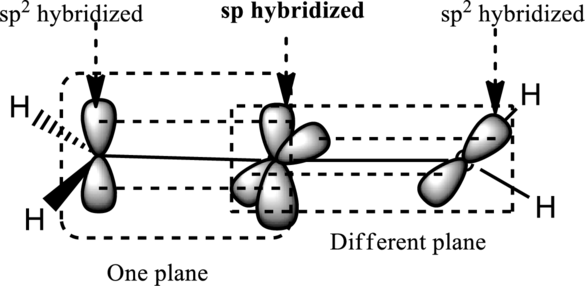
Thus the terminal hydrogen atom on carbon at position 1 is in a different plane than carbon at position 3 in case of
(f)
Interpretation:
Orientation of hydrogen atoms at end of chain has to be determined.
Concept Introduction:
In allene, the central carbon that is double bonded to each carbon in different planes is
(f)
Explanation of Solution
For structures with even number of carbon atoms, for example, consider the structure:
Here, the relative position of the hydrogen atom is indicated as follows:
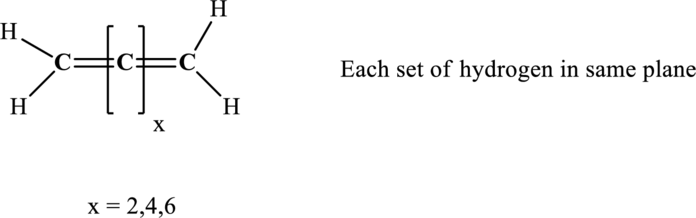
For structures with an odd number of carbon atoms, for example, consider the structure:
Here, the relative position of the hydrogen atom is indicated as follows:

Thus structures with even a number of carbon atoms as in
Want to see more full solutions like this?
Chapter 2 Solutions
ACHIEVE/CHEMICAL PRINCIPLES ACCESS 2TERM
- For Raman spectroscopy/imaging, which statement is not true regarding its disadvantages? a) Limited spatial resolution. b) Short integration time. c) A one-dimensional technique. d) Weak signal, only 1 in 108 incident photons is Raman scattered. e) Fluorescence interference.arrow_forwardUsing a cell of known pathlength b = 1.25115 x 10-3 cm, a water absorption spectrum was measured. The band at 1645 cm-1, assigned to the O-H bending, showed an absorbance, A, of 1.40. a) Assuming that water density is 1.00 g/mL, calculate the water molar concentration c (hint: M= mole/L) b) Calculate the molar absorptivity, a, of the 1645 cm-1 band c) The transmitted light, I, can be written as I= Ioexp(-xb), where x is the absorption coefficient (sometimes designated as alpha), Io is the input light, and b is the cell pathlength. Prove that x= (ln10)*x*c. (Please provide a full derivation of the equation for x from the equation for I). d) Calculate x for the 1645 cm-1 bandarrow_forwardI need help with the follloaingarrow_forward
- For a CARS experiment on a Raman band 918 cm-1, if omega1= 1280 nm, calculate the omega2 in wavelength (nm) and the CARS output in wavelength (nm).arrow_forwardI need help with the following questionarrow_forwardFor CARS, which statement is not true regarding its advantages? a) Contrast signal based on vibrational characteristics, no need for fluorescent tagging. b) Stronger signals than spontaneous Raman. c) Suffers from fluorescence interference, because CARS signal is at high frequency. d) Faster, more efficient imaging for real-time analysis. e) Higher resolution than spontaneous Raman microscopy.arrow_forward
- Draw the major product of the Claisen condensation reaction between two molecules of this ester. Ignore inorganic byproducts. Incorrect, 5 attempts remaining 1. NaOCH3/CH3OH 2. Acidic workup Select to Draw O Incorrect, 5 attempts remaining The total number of carbons in the parent chain is incorrect. Review the reaction conditions including starting materials and/or intermediate structures and recount the number of carbon atoms in the parent chain of your structure. OKarrow_forwardUsing a cell of known pathlength b = 1.25115 x 10-3 cm, a water absorption spectrum was measured. The band at 1645 cm-1, assigned to the O-H bending, showed an absorbance, A, of 1.40. a) Assuming that water density is 1.00 g/mL, calculate the water molar concentration c (hint: M= mole/L) b) Calculate the molar absorptivity, a, of the 1645 cm-1 band c) The transmitted light, I, can be written as I= Ioexp(-xb), where x is the absorption coefficient (sometimes designated as alpha), Io is the input light, and b is the cell pathlength. Prove that x= (ln10)*x*c d) Calculate x for the 1645 cm-1 bandarrow_forwardConvert 1.38 eV into wavelength (nm) and wavenumber (cm-1) (c = 2.998 x 108 m/s; h = 6.626 x 10-34 J*s).arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning


