
Concept explainers
(a)
Interpretation:
Possible mechanism for the given reaction has to be proposed.
Concept Introduction:
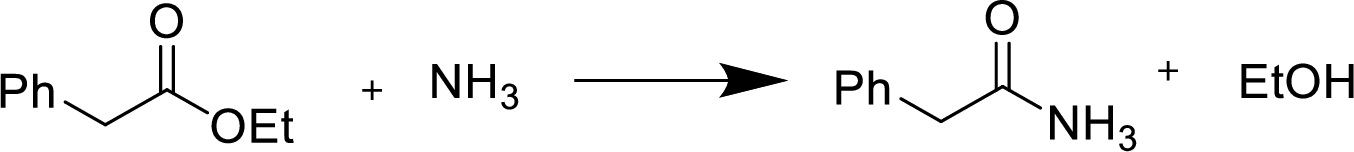
Reaction of an ester with ammonia or an
Treatment of an ester with ammonia or a primary or secondary amine gives an amide.
The nucleophilic addition of the ammonia or amine to the carbonyl carbon occurs followed by a proton transfer and a tetrahedral addition intermediate is formed. The intermediate can directly alkoxide and lose a proton to the alkoxide to give products.
(b)
Interpretation:
More acidic has to be identified in the given barbital molecule and the acidity of this molecule has to be accounted.
Concept Introduction:
Where,
If we know which proton is the more acidic and which proton is the less acidic we can make the determination regarding basicity.
Trending nowThis is a popular solution!

Chapter 18 Solutions
Organic Chemistry
- What is the name of the following compound? SiMe3arrow_forwardK Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


