
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Chapter 18, Problem 18.21P
Interpretation Introduction
Interpretation:
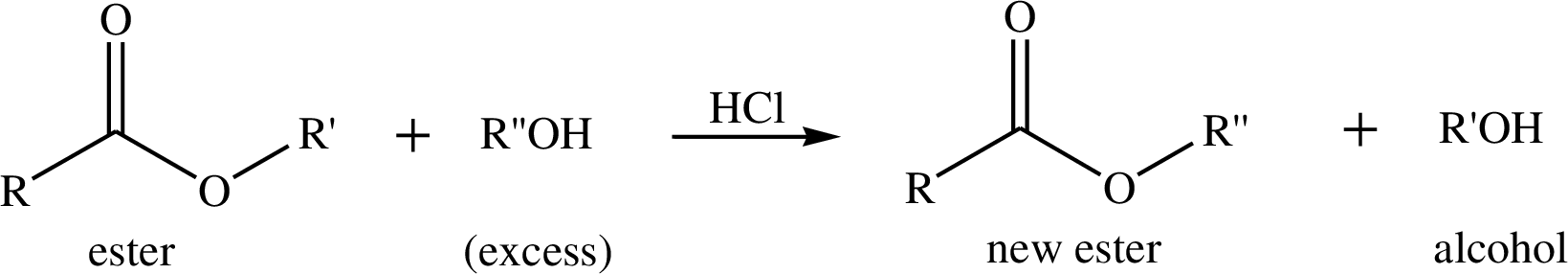
Way in which the given observation bears on the formation of a terahedral carbonyl addition intermediate during acid-catalysed easter hydrolyis, the corresponding transformation has to be shown.
Concept Introduction:
Transesterification is the process of formation of a new ester molecule from the reaction of alcohol and an ester. This is like hydrolysis of ester but here nucleophile is alcohol molecule instead of
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
What is the [OH⁻] of a 1.80 M solution of pyridine (C₅H₅N, Kb = 1.70 × 10⁻⁹)?
What is the percent ionization in a 0.260 M solution of formic acid (HCOOH) (Ka = 1.78 × 10⁻⁴)?
Determine the pH of solution of HC3H5O2 By constructing an ICE table writing the equilibrium constant expression, and using this information to determine the pH. The Ka of HC3H5O2 is 1.3 x 10-5
Chapter 18 Solutions
Organic Chemistry
Ch. 18.1 - Prob. 18.1PCh. 18.2 - Prob. 18.2PCh. 18.4 - Prob. 18.3PCh. 18.4 - Prob. 18.4PCh. 18.4 - Synthesis of nitriles by nucleophilic displacement...Ch. 18.5 - Complete the following transesterification...Ch. 18.6 - Complete and balance equations for the following...Ch. 18.8 - Prob. AQCh. 18.8 - Several compounds have been found to inhibit...Ch. 18.8 - Prob. CQ
Ch. 18.8 - The following sequence of steps is used to create...Ch. 18.9 - Prob. 18.8PCh. 18.9 - Prob. 18.9PCh. 18.10 - Prob. 18.10PCh. 18.10 - Show how to convert (R)-2-phenylpropanoic acid to...Ch. 18 - Prob. 18.12PCh. 18 - Write the IUPAC name for each compound. (a)...Ch. 18 - Prob. 18.14PCh. 18 - Prob. 18.15PCh. 18 - Propose a structural formula for compound A,...Ch. 18 - Propose a structural formula for compound B,...Ch. 18 - Propose a structural formula for each compound...Ch. 18 - Draw a structural formula for the principal...Ch. 18 - Prob. 18.20PCh. 18 - Prob. 18.21PCh. 18 - Prob. 18.22PCh. 18 - Prob. 18.23PCh. 18 - Prob. 18.24PCh. 18 - Show the product expected when the following...Ch. 18 - The reagent diisobutylaluminum hydride (DIBALH)...Ch. 18 - Prob. 18.27PCh. 18 - Prob. 18.28PCh. 18 - Prob. 18.29PCh. 18 - Nicotinic acid, more commonly named niacin, is one...Ch. 18 - Prob. 18.31PCh. 18 - Prob. 18.32PCh. 18 - Prob. 18.33PCh. 18 - Prob. 18.34PCh. 18 - Prob. 18.35PCh. 18 - Isoniazid, a drug used to treat tuberculosis, is...Ch. 18 - Prob. 18.37PCh. 18 - A step in a synthesis of PGE1 (prostaglandin E1,...Ch. 18 - Prob. 18.39PCh. 18 - Prob. 18.40PCh. 18 - Show how to synthesize 5-nonanone from...Ch. 18 - Prob. 18.42PCh. 18 - The following sequence of steps converts...Ch. 18 - Prob. 18.44PCh. 18 - Prob. 18.45PCh. 18 - Prob. 18.46PCh. 18 - Prob. 18.47PCh. 18 - Following is a retrosynthetic analysis for the...Ch. 18 - Prob. 18.50PCh. 18 - Given this retrosynthetic analysis, propose a...Ch. 18 - Prob. 18.52PCh. 18 - Prob. 18.53PCh. 18 - Prob. 18.54PCh. 18 - In Problem 7.28, we saw this step in Johnsons...Ch. 18 - Prob. 18.56PCh. 18 - Prob. 18.57PCh. 18 - Prob. 18.58PCh. 18 - Prob. 18.59PCh. 18 - Prob. 18.60PCh. 18 - Prob. 18.61PCh. 18 - Prob. 18.62PCh. 18 - Using your reaction roadmap as a guide, show how...Ch. 18 - Using your reaction roadmap as a guide, show how...Ch. 18 - Using your reaction roadmap as a guide, show how...Ch. 18 - Using your reaction roadmap as a guide, show how...Ch. 18 - Minoxidil is a molecule that causes hair growth in...Ch. 18 - Prob. 18.69PCh. 18 - Prob. 18.70PCh. 18 - Prob. 18.71P
Knowledge Booster
Similar questions
- Determine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction LiNO3arrow_forwardAn unknown weak acid with a concentration of 0.410 M has a pH of 5.600. What is the Ka of the weak acid?arrow_forward(racemic) 19.84 Using your reaction roadmaps as a guide, show how to convert 2-oxepanone and ethanol into 1-cyclopentenecarbaldehyde. You must use 2-oxepanone as the source of all carbon atoms in the target molecule. Show all reagents and all molecules synthesized along the way. & + EtOH H 2-Oxepanone 1-Cyclopentenecarbaldehydearrow_forward
- R₂ R₁ R₁ a R Rg Nu R₂ Rg R₁ R R₁₂ R3 R R Nu enolate forming R₁ R B-Alkylated carbonyl species or amines Cyclic B-Ketoester R₁₁ HOB R R₁B R R₁₂ B-Hydroxy carbonyl R diester R2 R3 R₁ RB OR R₂ 0 aB-Unsaturated carbonyl NaOR Aldol HOR reaction 1) LDA 2) R-X 3) H₂O/H₂O ketone, aldehyde 1) 2°-amine 2) acid chloride 3) H₂O'/H₂O 0 O R₁ R₁ R R₁ R₁₂ Alkylated a-carbon R₁ H.C R₁ H.C Alkylated methyl ketone acetoacetic ester B-Ketoester ester R₁ HO R₂ R B-Dicarbonyl HO Alkylated carboxylic acid malonic ester Write the reagents required to bring about each reaction next to the arrows shown. Next, record any regiochemistry or stereochemistry considerations relevant to the reaction. You should also record any key aspects of the mechanism, such as forma- tion of an important intermediate, as a helpful reminder. You may want to keep track of all reactions that make carbon-carbon bonds, because these help you build large molecules from smaller fragments. This especially applies to the reactions in…arrow_forwardProvide the reasonable steps to achieve the following synthesis.arrow_forwardIdentify which compound is more acidic. Justify your choice.arrow_forward
- Provide the reasonable steps to achieve the following synthesis.arrow_forwardWhen anisole is treated with excess bromine, the reaction gives a product which shows two singlets in 1H NMR. Draw the product.arrow_forward(ii) Draw a reasonable mechanism for the following reaction: CI NaOH heat OH (hint: SNAr Reaction) :arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning
