
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 18.4, Problem 18.5P
Synthesis of nitriles by nucleophilic displacement of halide from a haloalkane (also called an
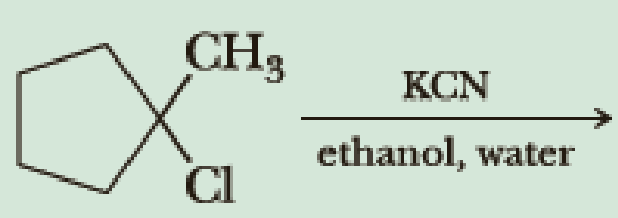
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
An einstein is the amount of energy needed to dissociate 1 mole of a substance. If we have 0.58 moles, do we need 0.58 einsteins to dissociate that substance?
If the energy absorbed per mole of gas is 480 kJ mol-1, indicate the number of Einsteins per mole.Data: Energy of each photon: 0.7835x10-18 J.
If the energy absorbed per mole of gas is 480 kJ mol-1, indicate the number of Einsteins per mole.
Chapter 18 Solutions
Organic Chemistry
Ch. 18.1 - Prob. 18.1PCh. 18.2 - Prob. 18.2PCh. 18.4 - Prob. 18.3PCh. 18.4 - Prob. 18.4PCh. 18.4 - Synthesis of nitriles by nucleophilic displacement...Ch. 18.5 - Complete the following transesterification...Ch. 18.6 - Complete and balance equations for the following...Ch. 18.8 - Prob. AQCh. 18.8 - Several compounds have been found to inhibit...Ch. 18.8 - Prob. CQ
Ch. 18.8 - The following sequence of steps is used to create...Ch. 18.9 - Prob. 18.8PCh. 18.9 - Prob. 18.9PCh. 18.10 - Prob. 18.10PCh. 18.10 - Show how to convert (R)-2-phenylpropanoic acid to...Ch. 18 - Prob. 18.12PCh. 18 - Write the IUPAC name for each compound. (a)...Ch. 18 - Prob. 18.14PCh. 18 - Prob. 18.15PCh. 18 - Propose a structural formula for compound A,...Ch. 18 - Propose a structural formula for compound B,...Ch. 18 - Propose a structural formula for each compound...Ch. 18 - Draw a structural formula for the principal...Ch. 18 - Prob. 18.20PCh. 18 - Prob. 18.21PCh. 18 - Prob. 18.22PCh. 18 - Prob. 18.23PCh. 18 - Prob. 18.24PCh. 18 - Show the product expected when the following...Ch. 18 - The reagent diisobutylaluminum hydride (DIBALH)...Ch. 18 - Prob. 18.27PCh. 18 - Prob. 18.28PCh. 18 - Prob. 18.29PCh. 18 - Nicotinic acid, more commonly named niacin, is one...Ch. 18 - Prob. 18.31PCh. 18 - Prob. 18.32PCh. 18 - Prob. 18.33PCh. 18 - Prob. 18.34PCh. 18 - Prob. 18.35PCh. 18 - Isoniazid, a drug used to treat tuberculosis, is...Ch. 18 - Prob. 18.37PCh. 18 - A step in a synthesis of PGE1 (prostaglandin E1,...Ch. 18 - Prob. 18.39PCh. 18 - Prob. 18.40PCh. 18 - Show how to synthesize 5-nonanone from...Ch. 18 - Prob. 18.42PCh. 18 - The following sequence of steps converts...Ch. 18 - Prob. 18.44PCh. 18 - Prob. 18.45PCh. 18 - Prob. 18.46PCh. 18 - Prob. 18.47PCh. 18 - Following is a retrosynthetic analysis for the...Ch. 18 - Prob. 18.50PCh. 18 - Given this retrosynthetic analysis, propose a...Ch. 18 - Prob. 18.52PCh. 18 - Prob. 18.53PCh. 18 - Prob. 18.54PCh. 18 - In Problem 7.28, we saw this step in Johnsons...Ch. 18 - Prob. 18.56PCh. 18 - Prob. 18.57PCh. 18 - Prob. 18.58PCh. 18 - Prob. 18.59PCh. 18 - Prob. 18.60PCh. 18 - Prob. 18.61PCh. 18 - Prob. 18.62PCh. 18 - Using your reaction roadmap as a guide, show how...Ch. 18 - Using your reaction roadmap as a guide, show how...Ch. 18 - Using your reaction roadmap as a guide, show how...Ch. 18 - Using your reaction roadmap as a guide, show how...Ch. 18 - Minoxidil is a molecule that causes hair growth in...Ch. 18 - Prob. 18.69PCh. 18 - Prob. 18.70PCh. 18 - Prob. 18.71P
Additional Science Textbook Solutions
Find more solutions based on key concepts
Describe Mendels conclusions about how traits are passed from generation to generation.
Concepts of Genetics (12th Edition)
Give the IUPAC name for each compound.
Organic Chemistry
11. In the early 1800s, French naturalist Jean Baptiste Lamarck suggested that the best explanation for the rel...
Campbell Biology: Concepts & Connections (9th Edition)
Why is it unlikely that two neighboring water molecules would be arranged like this?
Campbell Biology (11th Edition)
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The quantum yield of the photochemical decay of HI is 2. Calculating the moles of HI per kJ of radiant energy can be decayed knowing that the energy absorbed per mole of photons is 490 kJ.arrow_forwardThe quantum yield of the photochemical decay of HI is 2. Calculate the number of Einsteins absorbed per mole knowing that the energy absorbed per mole of photons is 490 kJ.arrow_forwardThe quantum yield of the photochemical decay of HI is 2. How many moles of HI per kJ of radiant energy can be decayed knowing that the energy absorbed per mole of photons is 490 kJ.arrow_forward
- If the energy absorbed per mole of photons is 450 kJ, the number of Einsteins absorbed per 1 mole.arrow_forwardWhen propionic aldehyde in vapor form at 200 mmHg and 30°C is irradiated with radiation of wavelength 302 nm, the quantum yield with respect to the formation of CO is 0.54. If the intensity of the incident radiation is 1.5x10-3 W, find the rate of formation of CO.arrow_forwardDraw mechanismarrow_forward
- Does Avogadro's number have units?arrow_forwardExplain why the total E in an Einstein depends on the frequency or wavelength of the light.arrow_forwardIf the dissociation energy of one mole of O2 is 5.17 eV, determine the wavelength that must be used to dissociate it with electromagnetic radiation. Indicate how many Einstein's of this radiation are needed to dissociate 1 liter of O2 at 25°C and 1 atm of pressure.Data: 1 eV = 96485 kJ mol-1; R = 0.082 atm L K-1; c = 2.998x108 m s-1; h = 6.626x10-34 J s; NA = 6.022x 1023 mol-1arrow_forward
- Indicate the number of Einsteins that are equivalent to 550 kJ mol⁻¹ of absorbed energy (wavelength 475 nm).arrow_forwardIndicate the number of einsteins that are equivalent to 550 kJ mol⁻¹ of absorbed energy?arrow_forwardA unit used in photochemistry is the einstein. If 400 kJ mol-1 of energy has been absorbed, how many einsteins is this equivalent to?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole

Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY