
(a)
Interpretation:
The way by which tolazamide is synthesized has to be shown.
Concept introduction:
Reaction of an ester with ammonia or an
Treatment of an ester with ammonia or a primary or secondary amine gives an amide.
The nucleophilic addition of the ammonia or amine to the carbonyl carbon occurs followed by a proton transfer and a tetrahedral addition intermediate is formed. The intermediate can directly alkoxide and lose a proton to the alkoxide to give products.
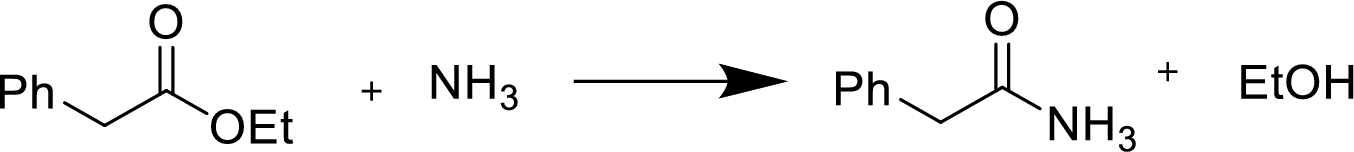
(b)
Interpretation:
The way by which Gliclazide is synthesized has to be shown.
Concept introduction:
Reaction of an ester with ammonia or an amine:
Treatment of an ester with ammonia or a primary or secondary amine gives an amide.
The nucleophilic addition of the ammonia or amine to the carbonyl carbon occurs followed by a proton transfer and a tetrahedral addition intermediate is formed. The intermediate can directly alkoxide and lose a proton to the alkoxide to give products.
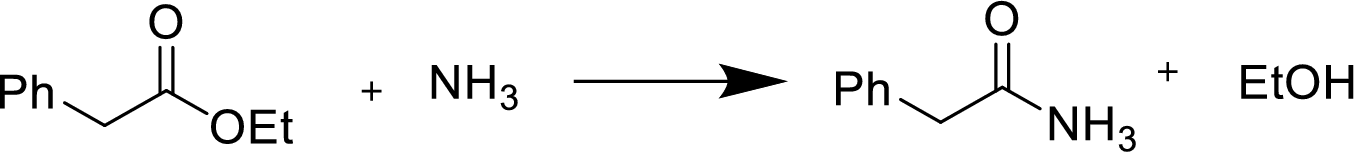
Want to see the full answer?
Check out a sample textbook solution
Chapter 18 Solutions
Organic Chemistry
- Curved arrows were used to generate the significant resonance structure and labeled the most significant contribute. What are the errors in these resonance mechanisms. Draw out the correct resonance mechanisms with an brief explanation.arrow_forwardWhat are the: нсе * Moles of Hice while given: a) 10.0 ml 2.7M ? 6) 10.ome 12M ?arrow_forwardYou are asked to use curved arrows to generate the significant resonance structures for the following series of compounds and to label the most significant contributor. Identify the errors that would occur if you do not expand the Lewis structures or double-check the mechanisms. Also provide the correct answers.arrow_forward
- how to get limiting reactant and % yield based off this data Compound Mass 6) Volume(mL Ben zaphone-5008 ne Acetic Acid 1. Sam L 2-propanot 8.00 Benzopin- a col 030445 Benzopin a Colone 0.06743 Results Compound Melting Point (°c) Benzopin acol 172°c - 175.8 °c Benzoping to lone 1797-180.9arrow_forwardAssign ALL signals for the proton and carbon NMR spectra on the following pages.arrow_forward7.5 1.93 2.05 C B A 4 3 5 The Joh. 9 7 8 1 2 7.5 7.0 6.5 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 ppm 9 7 8 0.86 OH 10 4 3 5 1 2 7.5 7.0 6.5 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 ppm 9 7 8 CI 4 3 5 1 2 7.0 6.5 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 2.21 4.00 1.5 2.00 2.07 1.0 ppm 2.76arrow_forward
- Assign the functional group bands on the IR spectra.arrow_forwardFind the pH of a 0.120 M solution of HNO2. Find the pH ignoring activity effects (i.e., the normal way). Find the pH in a solution of 0.050 M NaCl, including activityarrow_forwardPlease help me answer these three questions. Required info should be in data table.arrow_forward
- Draw the major organic substitution product or products for (2R,3S)-2-bromo-3-methylpentane reacting with the given nucleophile. Clearly drawn the stereochemistry, including a wedged bond, a dashed bond and two in-plane bonds at each stereogenic center. Omit any byproducts. Bri CH3CH2O- (conc.) Draw the major organic product or products.arrow_forwardTartaric acid (C4H6O6) is a diprotic weak acid. A sample of 875 mg tartaric acid are dissolved in 100 mL water and titrated with 0.994 M NaOH. How many mL of NaOH are needed to reach the first equivalence point? How many mL of NaOH are needed to reach the second equivalence point?arrow_forwardIncluding activity, calculate the solubility of Pb(IO3)2 in a matrix of 0.020 M Mg(NO3)2.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning



