
Organic Chemistry (8th Edition)
8th Edition
ISBN: 9780134042282
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 16.4, Problem 5P
What products are formed when the following compounds react with CH3MgBr, followed by the addition of dilute acid? Disregard stereoisomers.
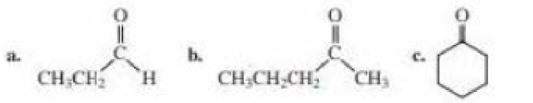
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
All of these compounds would be produced (I think). In my book, I don't see any rules about yield in this case, like explaining that one product would be present in less yield for this reason or that reason. Please explain why some of these produce less yield than others.
5. Fill in the missing molecules in the following reaction pathway.
TMSO
Heat
+
CI
then HF
O₂N
(1.0 equiv)
AICI 3
OMe
e.
O₂N
NO2
1. excess H2, Pd/C
2. excess NaNO2, HCI
3. excess CuCN
Chapter 16 Solutions
Organic Chemistry (8th Edition)
Ch. 16.1 - Give two names for each of the following:Ch. 16.1 - Prob. 2PCh. 16.1 - Name the following:Ch. 16.2 - Prob. 4PCh. 16.4 - What products are formed when the following...Ch. 16.4 - We saw on the previous page that...Ch. 16.4 - a. How many stereoisomers are obtained from the...Ch. 16.4 - Prob. 9PCh. 16.4 - Write the mechanism for the reaction of acetyl...Ch. 16.4 - Prob. 11P
Ch. 16.4 - Show how the following compounds can be...Ch. 16.4 - Prob. 13PCh. 16.4 - Prob. 14PCh. 16.4 - In the mechanism for cyanohydrin formation, why is...Ch. 16.4 - Prob. 16PCh. 16.4 - Prob. 17PCh. 16.4 - Show two ways to convert an alkyl halide into a...Ch. 16.5 - Prob. 20PCh. 16.5 - Prob. 21PCh. 16.5 - Prob. 22PCh. 16.5 - Prob. 23PCh. 16.6 - Prob. 24PCh. 16.7 - What reducing agents should be used to obtain the...Ch. 16.7 - Prob. 26PCh. 16.8 - Prob. 27PCh. 16.8 - Prob. 28PCh. 16.8 - Prob. 29PCh. 16.8 - The pKa of protonated acetone is about 7.5. and...Ch. 16.8 - Prob. 31PCh. 16.8 - Prob. 32PCh. 16.8 - Prob. 33PCh. 16.8 - Excess ammonia must be used when a primary amine...Ch. 16.8 - The compounds commonly known as amino acids are...Ch. 16.9 - Hydration of an aldehyde is also catalyzed by...Ch. 16.9 - Which ketone forms the most hydrate in an aqueous...Ch. 16.9 - When trichloroacetaldehyde is dissolved in water,...Ch. 16.9 - Which of the following are a. hermiacetals? b....Ch. 16.9 - Prob. 40PCh. 16.9 - Explain why an acetal can be isolated but most...Ch. 16.10 - Prob. 42PCh. 16.10 - Prob. 43PCh. 16.10 - What products would be formed from the proceedings...Ch. 16.10 - a. In a six-step synthesis, what is the yield of...Ch. 16.10 - Show how each of the following compounds could be...Ch. 16.12 - Prob. 47PCh. 16.13 - Prob. 49PCh. 16.14 - Prob. 50PCh. 16.15 - Prob. 51PCh. 16.16 - Prob. 52PCh. 16 - Draw the structure for each of the following: a....Ch. 16 - Prob. 54PCh. 16 - Prob. 55PCh. 16 - Prob. 56PCh. 16 - a. Show the reagents required to form the primary...Ch. 16 - Prob. 58PCh. 16 - Prob. 59PCh. 16 - Using cyclohexanone as the starting material,...Ch. 16 - Propose a mechanism for each of the following...Ch. 16 - Show how each of the following compounds can be...Ch. 16 - Fill in the boxes:Ch. 16 - Prob. 64PCh. 16 - Identify A through O:Ch. 16 - Prob. 66PCh. 16 - Prob. 67PCh. 16 - Prob. 68PCh. 16 - How many signals would the product of the...Ch. 16 - Prob. 70PCh. 16 - Prob. 71PCh. 16 - Prob. 72PCh. 16 - Prob. 73PCh. 16 - Prob. 74PCh. 16 - Prob. 75PCh. 16 - Prob. 76PCh. 16 - Prob. 77PCh. 16 - Prob. 78PCh. 16 - Draw structure for A-D for each of the following:Ch. 16 - Prob. 80PCh. 16 - a. Propose a mechanism for the following reaction:...Ch. 16 - Prob. 82PCh. 16 - A compound gives the following IR spectrum. Upon...Ch. 16 - How can be following compounds be prepared from...Ch. 16 - Prob. 85PCh. 16 - Prob. 86PCh. 16 - Prob. 87PCh. 16 - In the presence of an acid catalyst, acetaldehyde...Ch. 16 - Prob. 89PCh. 16 - Prob. 90PCh. 16 - Prob. 91PCh. 16 - A compound reacts with methylmagnesium bromide...Ch. 16 - Show how each of the following compounds can be...Ch. 16 - Prob. 94PCh. 16 - The pKa values of the carboxylic acid groups of...Ch. 16 - The Baylis-Hillman reaction is a DABCO...Ch. 16 - Prob. 97PCh. 16 - Prob. 98P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Help with a periodic table task.' Procedure Part 1: Customizing a Periodic Table Use a textbook or other valid source to determine which elements are metals, nonmetals, metalloids (called semimetals in some texts), alkali metals, alkaline earth metals, transition metals, halogens, and noble gases. Download and print a copy of the Periodic Table of Elements. Use colored pencils, colorful highlighters, or computer drawing tools to devise a schematic for designating each of the following on the periodic table: Group numbers Period number Labels for these groups: alkali metals, alkaline earth metals, transition metals, inner transition metals (lanthanides and actinides), other metals, metalloids (semimetals), other nonmetals, halogens, and noble gases Metals, nonmetals, and metalloids Note: Write the group and period numbers and color/highlight each element for categorization. Be sure to include a key for the schematic. Take a photo of the completed periodic table and upload the…arrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forwardCan you explain these two problems for mearrow_forward
- 个 ^ Blackboard x Organic Chemistry II Lecture (m x Aktiv Learning App x → C app.aktiv.com ← Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Problem 28 of 35 :OH H HH KO Select to Edit Arrows CH CH₂OK, CH CH2OH 5+ H :0: Donearrow_forwardCan you explain those two problems for me please.arrow_forwardDo we need to draw the "ethyne" first for this problem? im confusedarrow_forward
- Can you explain how this problem was solved.arrow_forwardQuestion 2 show work. don't Compound give Ai generated solution So (J K-1 mol-1) A 26 B 54 C 39 D 49 At 298 K, AG° is 375 kJ for the reaction 1A + 1B → 4C + 2D Calculate AH° for this reaction in kJ.arrow_forward1. Provide a complete IUPAC name for each of the following compounds. a) b) c) OH OH OH a) b) c) 2. Provide a complete IUPAC name for each of the following compounds. a) b) a) OH b) он c) OB >=arrow_forward
- c) 3. Provide a common name for each of the following alcohols. a) a) OH b) OH c) HO b) c) 4. Provide a common name for each of the following compounds. b) OH a) 5 a) Y OH c) OHarrow_forwardUsing the critical constants for water (refer to the table in the lecture slides), calculate the second virial coefficient. Assume that the compression factor (Z) is expressed as an expansion series in terms of pressure.arrow_forward+3413 pts /4800 Question 38 of 48 > Write the full electron configuration for a Kion. © Macmillan Learning electron configuration: ↓ Resources Solution Penalized → Al Tutor Write the full electron configuration for an Fion. electron configuration: T G 6 & 7 Y H כ Y 00 8 hp 9 J K no L 144 P 112 | t KC 47°F Clear ins prt sc delete ] backspace erarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning
How to Design a Total Synthesis; Author: Chemistry Unleashed;https://www.youtube.com/watch?v=9jRfAJJO7mM;License: Standard YouTube License, CC-BY