
Organic Chemistry (8th Edition)
8th Edition
ISBN: 9780134042282
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 16, Problem 69P
How many signals would the product of the following reaction show in
- a. its 1H NMR spectrum?
- b. its 13C NMR spectrum?
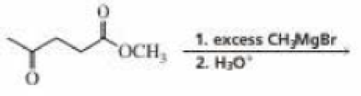
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
When talking about the acidity of carboxylic acids, is it the same thing to say higher or stronger acidity?
Using the following two half-reactions, determine the pH range in which $NO_2^-\ (aq)$ cannot be found as the predominant chemical species in water.* $NO_3^-(aq)+10H^+(aq)+8e^-\rightarrow NH_4^+(aq)+3H_2O(l),\ pE^{\circ}=14.88$* $NO_2^-(aq)+8H^+(aq)+6e^-\rightarrow NH_4^+(aq)+2H_2O(l),\ pE^{\circ}=15.08$
Indicate characteristics of oxodec acid.
Chapter 16 Solutions
Organic Chemistry (8th Edition)
Ch. 16.1 - Give two names for each of the following:Ch. 16.1 - Prob. 2PCh. 16.1 - Name the following:Ch. 16.2 - Prob. 4PCh. 16.4 - What products are formed when the following...Ch. 16.4 - We saw on the previous page that...Ch. 16.4 - a. How many stereoisomers are obtained from the...Ch. 16.4 - Prob. 9PCh. 16.4 - Write the mechanism for the reaction of acetyl...Ch. 16.4 - Prob. 11P
Ch. 16.4 - Show how the following compounds can be...Ch. 16.4 - Prob. 13PCh. 16.4 - Prob. 14PCh. 16.4 - In the mechanism for cyanohydrin formation, why is...Ch. 16.4 - Prob. 16PCh. 16.4 - Prob. 17PCh. 16.4 - Show two ways to convert an alkyl halide into a...Ch. 16.5 - Prob. 20PCh. 16.5 - Prob. 21PCh. 16.5 - Prob. 22PCh. 16.5 - Prob. 23PCh. 16.6 - Prob. 24PCh. 16.7 - What reducing agents should be used to obtain the...Ch. 16.7 - Prob. 26PCh. 16.8 - Prob. 27PCh. 16.8 - Prob. 28PCh. 16.8 - Prob. 29PCh. 16.8 - The pKa of protonated acetone is about 7.5. and...Ch. 16.8 - Prob. 31PCh. 16.8 - Prob. 32PCh. 16.8 - Prob. 33PCh. 16.8 - Excess ammonia must be used when a primary amine...Ch. 16.8 - The compounds commonly known as amino acids are...Ch. 16.9 - Hydration of an aldehyde is also catalyzed by...Ch. 16.9 - Which ketone forms the most hydrate in an aqueous...Ch. 16.9 - When trichloroacetaldehyde is dissolved in water,...Ch. 16.9 - Which of the following are a. hermiacetals? b....Ch. 16.9 - Prob. 40PCh. 16.9 - Explain why an acetal can be isolated but most...Ch. 16.10 - Prob. 42PCh. 16.10 - Prob. 43PCh. 16.10 - What products would be formed from the proceedings...Ch. 16.10 - a. In a six-step synthesis, what is the yield of...Ch. 16.10 - Show how each of the following compounds could be...Ch. 16.12 - Prob. 47PCh. 16.13 - Prob. 49PCh. 16.14 - Prob. 50PCh. 16.15 - Prob. 51PCh. 16.16 - Prob. 52PCh. 16 - Draw the structure for each of the following: a....Ch. 16 - Prob. 54PCh. 16 - Prob. 55PCh. 16 - Prob. 56PCh. 16 - a. Show the reagents required to form the primary...Ch. 16 - Prob. 58PCh. 16 - Prob. 59PCh. 16 - Using cyclohexanone as the starting material,...Ch. 16 - Propose a mechanism for each of the following...Ch. 16 - Show how each of the following compounds can be...Ch. 16 - Fill in the boxes:Ch. 16 - Prob. 64PCh. 16 - Identify A through O:Ch. 16 - Prob. 66PCh. 16 - Prob. 67PCh. 16 - Prob. 68PCh. 16 - How many signals would the product of the...Ch. 16 - Prob. 70PCh. 16 - Prob. 71PCh. 16 - Prob. 72PCh. 16 - Prob. 73PCh. 16 - Prob. 74PCh. 16 - Prob. 75PCh. 16 - Prob. 76PCh. 16 - Prob. 77PCh. 16 - Prob. 78PCh. 16 - Draw structure for A-D for each of the following:Ch. 16 - Prob. 80PCh. 16 - a. Propose a mechanism for the following reaction:...Ch. 16 - Prob. 82PCh. 16 - A compound gives the following IR spectrum. Upon...Ch. 16 - How can be following compounds be prepared from...Ch. 16 - Prob. 85PCh. 16 - Prob. 86PCh. 16 - Prob. 87PCh. 16 - In the presence of an acid catalyst, acetaldehyde...Ch. 16 - Prob. 89PCh. 16 - Prob. 90PCh. 16 - Prob. 91PCh. 16 - A compound reacts with methylmagnesium bromide...Ch. 16 - Show how each of the following compounds can be...Ch. 16 - Prob. 94PCh. 16 - The pKa values of the carboxylic acid groups of...Ch. 16 - The Baylis-Hillman reaction is a DABCO...Ch. 16 - Prob. 97PCh. 16 - Prob. 98P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the final product when hexanedioic acid reacts with 1º PCl5 and 2º NH3.arrow_forwardWhat is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forward
- The temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY