
Organic Chemistry (8th Edition)
8th Edition
ISBN: 9780134042282
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 16.1, Problem 1P
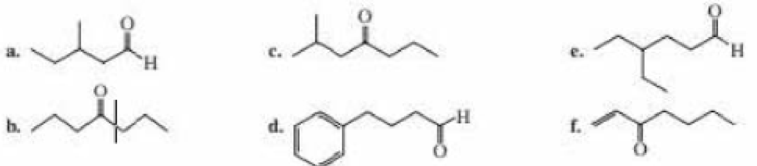
Give two names for each of the following:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
What is the significance of selecting a "representative" sample for chemical analysis, and how does this practice ensure accurate and reliable results with respect to chemical analyses?
Identify and provide an explanation of the differences between homogeneous and heterogeneous sampling in the context of sampling methods.
Г
C-RSA CHROMATOPAC CH=1
DATA 1: @CHRM1.C00
ATTEN=10 SPEED= 10.0
0.0 b.092
0.797
1.088
1.813
C-RSA CHROMATOPAC CH=1 Report No. =13
** CALCULATION REPORT **
DATA=1: @CHRM1.000 11/03/05 08:09:52
CH PKNO
TIME
1
2
0.797
3
1.088
4
1.813
AREA
1508566
4625442
2180060
HEIGHT
207739
701206 V
287554 V
MK IDNO
CONC
NAME
18.1447
55.6339
26.2213
TOTAL
8314067 1196500
100
C-R8A CHROMATOPAC CH=1
DATA 1: @CHRM1.C00
ATTEN=10 SPEED= 10.0
0. 0
087
337.
0.841
1.150
C-R8A CHROMATOPAC CH=1 Report No. =14
DATA=1: @CHRM1.000 11/03/05 08:12:40
** CALCULATION REPORT **
CH PKNO
TIME
AREA
1
3 0.841
1099933
41.15
4039778
HEIGHT MK IDNO
170372
649997¯¯¯
CONC
NAME
21.4007
78.5993
TOTAL
5139711 820369
100
3
C-R8A CHROMATOPAC
CH=1
DATA 1: @CHRM1.C00
ATTEN=10 SPEED= 10.0
0.100
0:652
5.856
3
1.165
C-RSA CHROMATOPAC CH-1 Report No. =15
DATA=1: @CHRM1.000 11/03/05 08:15:26
** CALCULATION REPORT **
CH PKNO TIME
AREA
HEIGHT MK IDNO CONC
NAME
1 3 3 0.856
4
1.165
TOTAL
1253386
4838738
175481
708024 V
20.5739
79.4261
6092124…
Chapter 16 Solutions
Organic Chemistry (8th Edition)
Ch. 16.1 - Give two names for each of the following:Ch. 16.1 - Prob. 2PCh. 16.1 - Name the following:Ch. 16.2 - Prob. 4PCh. 16.4 - What products are formed when the following...Ch. 16.4 - We saw on the previous page that...Ch. 16.4 - a. How many stereoisomers are obtained from the...Ch. 16.4 - Prob. 9PCh. 16.4 - Write the mechanism for the reaction of acetyl...Ch. 16.4 - Prob. 11P
Ch. 16.4 - Show how the following compounds can be...Ch. 16.4 - Prob. 13PCh. 16.4 - Prob. 14PCh. 16.4 - In the mechanism for cyanohydrin formation, why is...Ch. 16.4 - Prob. 16PCh. 16.4 - Prob. 17PCh. 16.4 - Show two ways to convert an alkyl halide into a...Ch. 16.5 - Prob. 20PCh. 16.5 - Prob. 21PCh. 16.5 - Prob. 22PCh. 16.5 - Prob. 23PCh. 16.6 - Prob. 24PCh. 16.7 - What reducing agents should be used to obtain the...Ch. 16.7 - Prob. 26PCh. 16.8 - Prob. 27PCh. 16.8 - Prob. 28PCh. 16.8 - Prob. 29PCh. 16.8 - The pKa of protonated acetone is about 7.5. and...Ch. 16.8 - Prob. 31PCh. 16.8 - Prob. 32PCh. 16.8 - Prob. 33PCh. 16.8 - Excess ammonia must be used when a primary amine...Ch. 16.8 - The compounds commonly known as amino acids are...Ch. 16.9 - Hydration of an aldehyde is also catalyzed by...Ch. 16.9 - Which ketone forms the most hydrate in an aqueous...Ch. 16.9 - When trichloroacetaldehyde is dissolved in water,...Ch. 16.9 - Which of the following are a. hermiacetals? b....Ch. 16.9 - Prob. 40PCh. 16.9 - Explain why an acetal can be isolated but most...Ch. 16.10 - Prob. 42PCh. 16.10 - Prob. 43PCh. 16.10 - What products would be formed from the proceedings...Ch. 16.10 - a. In a six-step synthesis, what is the yield of...Ch. 16.10 - Show how each of the following compounds could be...Ch. 16.12 - Prob. 47PCh. 16.13 - Prob. 49PCh. 16.14 - Prob. 50PCh. 16.15 - Prob. 51PCh. 16.16 - Prob. 52PCh. 16 - Draw the structure for each of the following: a....Ch. 16 - Prob. 54PCh. 16 - Prob. 55PCh. 16 - Prob. 56PCh. 16 - a. Show the reagents required to form the primary...Ch. 16 - Prob. 58PCh. 16 - Prob. 59PCh. 16 - Using cyclohexanone as the starting material,...Ch. 16 - Propose a mechanism for each of the following...Ch. 16 - Show how each of the following compounds can be...Ch. 16 - Fill in the boxes:Ch. 16 - Prob. 64PCh. 16 - Identify A through O:Ch. 16 - Prob. 66PCh. 16 - Prob. 67PCh. 16 - Prob. 68PCh. 16 - How many signals would the product of the...Ch. 16 - Prob. 70PCh. 16 - Prob. 71PCh. 16 - Prob. 72PCh. 16 - Prob. 73PCh. 16 - Prob. 74PCh. 16 - Prob. 75PCh. 16 - Prob. 76PCh. 16 - Prob. 77PCh. 16 - Prob. 78PCh. 16 - Draw structure for A-D for each of the following:Ch. 16 - Prob. 80PCh. 16 - a. Propose a mechanism for the following reaction:...Ch. 16 - Prob. 82PCh. 16 - A compound gives the following IR spectrum. Upon...Ch. 16 - How can be following compounds be prepared from...Ch. 16 - Prob. 85PCh. 16 - Prob. 86PCh. 16 - Prob. 87PCh. 16 - In the presence of an acid catalyst, acetaldehyde...Ch. 16 - Prob. 89PCh. 16 - Prob. 90PCh. 16 - Prob. 91PCh. 16 - A compound reacts with methylmagnesium bromide...Ch. 16 - Show how each of the following compounds can be...Ch. 16 - Prob. 94PCh. 16 - The pKa values of the carboxylic acid groups of...Ch. 16 - The Baylis-Hillman reaction is a DABCO...Ch. 16 - Prob. 97PCh. 16 - Prob. 98P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the product of the reaction shown below. Ignore small byproducts that would evaporate please.arrow_forwardRelative Abundance 20- Problems 501 (b) The infrared spectrum has a medium-intensity peak at about 1650 cm. There is also a C-H out-of-plane bending peak near 880 cm. 100- 80- 56 41 69 M(84) LL 15 20 25 30 35 55 60 65 70 75 80 85 90 m/zarrow_forwardPolyethylene furanoate is a polymer made from plant-based sources; it is used for packaging. Identify the monomer(s) used in the production of this polymer using a condensation process.arrow_forward
- Phenol is the starting material for the synthesis of 2,3,4,5,6-pentachlorophenol, known al-ternatively as pentachlorophenol, or more simply as penta. At one time, penta was widely used as a wood preservative for decks, siding, and outdoor wood furniture. Draw the structural formula for pentachlorophenol and describe its synthesis from phenol.arrow_forward12 Mass Spectrometry (d) This unknown contains oxygen, but it does not show any significant infrared absorption peaks above 3000 cm . 59 100- BO 40 Relative Abundance M(102) - 15 20 25 30 35 40 45 50 5 60 65 70 75 80 85 90 95 100 105 mizarrow_forwardDraw a Haworth projection of a common cyclic form of this monosaccharide: H HO H HO H HO H H -OH CH2OH Click and drag to start drawing a structure. Х : Darrow_forward
- : Draw the structure of valylasparagine, a dipeptide made from valine and asparagine, as it would appear at physiological pH. Click and drag to start drawing a structure. P Darrow_forwardDraw the Haworth projection of α-L-mannose. You will find helpful information in the ALEKS Data resource. Click and drag to start drawing a structure. : ཊི Х Darrow_forwardDraw the structure of serine at pH 6.8. Click and drag to start drawing a structure. : d كarrow_forward
- Take a look at this molecule, and then answer the questions in the table below it. CH2OH H H H OH OH OH CH2OH H H H H OH H H OH H OH Is this a reducing sugar? yes α β ロ→ロ no ☑ yes Does this molecule contain a glycosidic bond? If you said this molecule does contain a glycosidic bond, write the symbol describing it. O no 0+0 If you said this molecule does contain a glycosidic bond, write the common names (including anomer and enantiomer labels) of the molecules that would be released if that bond were hydrolyzed. If there's more than one molecule, separate each name with a comma. ☐arrow_forwardAnswer the questions in the table below about this molecule: H₂N-CH₂ -C—NH–CH–C—NH–CH—COO- CH3 CH CH3 What kind of molecule is this? 0= CH2 C If you said the molecule is a peptide, write a description of it using 3-letter codes separated ☐ by dashes. polysaccharide peptide amino acid phospolipid none of the above Хarrow_forwardDraw a Haworth projection of a common cyclic form of this monosaccharide: CH₂OH C=O HO H H -OH H OH CH₂OH Click and drag to start drawing a structure. : ☐ Х S '☐arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY